Role of preoperative circulating tumor DNA in predicting occult metastases in resectable and borderline resectable pancreatic ductal adenocarcinoma
- PMID: 40900777
- PMCID: PMC12400235
- DOI: 10.3748/wjg.v31.i32.109383
Role of preoperative circulating tumor DNA in predicting occult metastases in resectable and borderline resectable pancreatic ductal adenocarcinoma
Abstract
Background: Some patients with resectable or borderline resectable pancreatic ductal adenocarcinoma (PDAC) may have distant metastases, undetected on preoperative imaging or early recurrence, within 6 months after surgery. Occult metastases (OMs) must be accurately predicted to optimize multidisciplinary treatment.
Aim: To investigate the efficacy of circulating tumor DNA (ctDNA) in predicting OM.
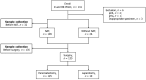
Methods: Two Japanese institutions prospectively collected preoperative plasma samples from PDAC patients between July 2019 and September 2021 and evaluated ctDNA using a targeted next-generation sequencing panel covering 52 cancer-related genes.
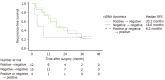
Results: Among 135 PDAC patients, 38 had OM and 35 were positive for ctDNA. The ctDNA positivity rate was significantly higher in patients with OM than in patients without OM. ctDNA-positive patients had significantly shorter median recurrence-free survival than ctDNA-negative patients. Logistic multivariate regression revealed ctDNA positivity as an independent predictor of OM.
Conclusion: Preoperative ctDNA in resectable PDAC is an independent predictor of OM and indicates poor prognosis following pancreatectomy and may be a useful biomarker in determining multidisciplinary patient care.
Keywords: Circulating tumor DNA; Early recurrence; Multidisciplinary treatment; Occult metastases; Pancreatic ductal adenocarcinoma.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures

References
-
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439–457. - PubMed
-
- Japan Pancreas Society. Classification of pancreatic carcinoma. 4th English ed. Kanehara and Co., Limited. 2017.
-
- Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05) Jpn J Clin Oncol. 2019;49:190–194. - PubMed
-
- Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

