MRS Imaging as Complement to MRI in the Post-treatment Follow-up of Glial Brain Tumors
- PMID: 40900884
- PMCID: PMC12401035
- DOI: 10.21873/cdp.10478
MRS Imaging as Complement to MRI in the Post-treatment Follow-up of Glial Brain Tumors
Abstract
Background/aim: Central nervous system tumors have a very low incidence worldwide. However, they represent a significant cause of mortality and morbidity. Magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS) and magnetic resonance spectroscopy imaging (MRSI) techniques provide metabolic information complementary to anatomical alterations. The aim of this study was to characterize different metabolic patterns and determine treatment outcomes in glial brain tumors.
Patients and methods: Forty-four previously treated patients participated in this prospective study, including 20 cases of low-grade (LG) and 24 high-grade (HG), gliomas. All patients underwent conventional MRI combined with MRS and MRSI using a 1.5 Tesla (T) magnet.
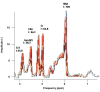
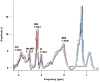
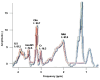
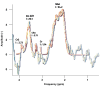
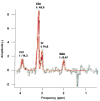
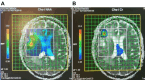
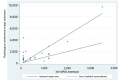
Results: Distinct metabolic profiles were observed via MRS and MRSI compared to normal brain tissue. Among the LG tumors, 10 remained stable with mean choline (Cho)/ N-Acetyl Aspartate (NAA) and NAA/creatine (Cr) ratios of 1.49 (p=0.036) and 0.92 (p=0.038), respectively, while the other 10 progressed to HG, with Cho/NAA and Cho/Cr ratios of 2.24 (p=0.026) and 4.48 (p=0.016). Among HG tumors, 17 remained stable with similar metabolic profiles, while seven showed progression. Gliosis was identified in 21 cases, characterized by a Cho/NAA ratio of 1.57 (p=0.028) and NAA/Cr ratio of 1.36 (p=0.026). Radiation necrosis was observed in 14 tumors, with significant spectroscopic changes including Cho/Cr ratios of 2.14 (p=0.02) and 1.9 (p=0.003), and NAA/Cr ratios of 1.28 (p=0.001) and 0.49 (p=0.001) across SV-MRS and MV-MRSI modalities. Tumor recurrence was detected in 20 cases based on MRSI metabolic maps.
Conclusion: MRS and MRSI provide valuable metabolic information that complements MRI in the post-treatment evaluation of glial brain tumors. These techniques enhance the detection of tumor recurrence, progression, and radiation necrosis, thereby supporting clinical decision-making and optimizing patient management.
Keywords: Brain tumors of glial origin; magnetic resonance spectroscopy; magnetic resonance spectroscopy imaging.
©2025 The Author(s). Published by the International Institute of Anticancer Research.
Conflict of interest statement
All Authors declare no competing interests regarding this study.
Figures
References
-
- Acosta M, Malaver G, Rodriguez C, Romero-Rojas A, Gamboa O, Arboleda G, Triana E, Zubieta C, Penagos P, Moreno-Acosta P. Magnetic resonance spectroscopic imaging in central nervous system brain tumors of glial origin. Rev Colomb Cancerol. 2022;26(2):150–163. doi: 10.35509/01239015.756. - DOI
LinkOut - more resources
Full Text Sources
