A combined model integrating deep learning, radiomics, and clinical ultrasound features for predicting BRAF V600E mutation in papillary thyroid carcinoma with Hashimoto's thyroiditis
- PMID: 40900897
- PMCID: PMC12399403
- DOI: 10.3389/fendo.2025.1641037
A combined model integrating deep learning, radiomics, and clinical ultrasound features for predicting BRAF V600E mutation in papillary thyroid carcinoma with Hashimoto's thyroiditis
Abstract
Objective: This study aims to develop an integrated model that combines radiomics, deep learning features, and clinical and ultrasound characteristics for predicting BRAF V600E mutations in patients with papillary thyroid carcinoma (PTC) combined with Hashimoto's thyroiditis (HT).
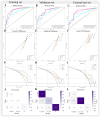
Methods: This retrospective study included 717 thyroid nodules from 672 patients with PTC combined with HT from four hospitals in China. Deep learning and radiomics were employed to extract deep learning and radiomics features from ultrasound images. Feature selection was performed using Pearson's correlation coefficient, the Minimum Redundancy Maximum Relevance (mRMR) algorithm, and LASSO regression. The optimal algorithm was selected from nine machine learning algorithms for model construction, including the traditional radiomics model (RAD), the deep learning model (DL), and their fusion model (DL_RAD). Additionally, a final combined model was developed by integrating the DL_RAD model with clinical and ultrasound features. Model performance was assessed using AUC, calibration curves, and decision curve analysis (DCA), while SHAP analysis was used to interpret the contribution of each feature to the combined model's output.
Results: The combined model achieved superior diagnostic performance, with AUC values of 0.895, 0.864, and 0.815 in the training, validation, and external test sets, respectively, outperforming the RAD model, DL model, and RAD_DL model. DeLong test results indicated significant differences in the external test set (p<0.05). Further validation through calibration curves and DCA confirmed the model's robust performance. SHAP analysis revealed that RAD_DL signature, aspect ratio, extrathyroidal extension, and gender were key contributors to the model's predictions.
Conclusion: The combined model integrating radiomics, deep learning features, and clinical as well as ultrasound characteristics exhibits excellent diagnostic performance in predicting BRAF V600E mutations in patients with PTC coexisting with HT, highlighting its strong potential for clinical application.
Keywords: BRAF V600E mutation; Hashimoto’s thyroiditis; deep learning; papillary thyroid carcinoma; radiomics; ultrasound.
Copyright © 2025 Zhu, Zhang, Zhou, Ben, Wang, Zeng, Cui and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission.
Figures




References
-
- Caulley L, Eskander A, Yang W, Auh E, Cairncross L, Cho NL, et al. Trends in diagnosis of noninvasive follicular thyroid neoplasm with papillarylike nuclear features and total thyroidectomies for patients with papillary thyroid neoplasms. JAMA Otolaryngol Head Neck Surg. (2022) 148:1–8. doi: 10.1001/jamaoto.2021.3277, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

