Identification of early predictors of clinical remission in primary membranous nephropathy: a post hoc analysis of the STARMEN trial
- PMID: 40900938
- PMCID: PMC12399971
- DOI: 10.1093/ckj/sfaf256
Identification of early predictors of clinical remission in primary membranous nephropathy: a post hoc analysis of the STARMEN trial
Abstract
Background: Patients with primary membranous nephropathy may progress to advanced chronic kidney disease despite immunosuppressive therapy (IST). Prediction of treatment response based on early and combined assessment of several standard clinical markers could improve risk stratification for progression, allowing timely individualization of treatment, which can optimize clinical outcomes and safety.
Methods: In this post hoc exploratory analysis of the STARMEN trial, we evaluated if combined baseline data, and IST-induced early changes in standard clinical markers predicted clinical remission at 2 years. The 2-year primary outcome was complete (CR) or partial remission (PR). The secondary outcome was CR. Additionally, we described kidney function outcomes. Standard clinical markers were incorporated into statistical modeling by logistic regression. Predictive performance was assessed by receiver operating characteristic curve analysis.
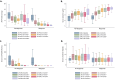
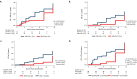
Results: The best multivariate model excluding immunosuppression to predict complete or PR at 2 years, included 3-month 24-h proteinuria, serum creatinine and immunological response [area under the curve (AUC) 0.87, 95% confidence interval (CI) 0.76-0.94, efficiency 87%]. For CR at 2 years, the best model included the same clinical markers at 6 months, but predictive performance was lower (AUC 0.74, 95% CI 0.62-0.85, efficiency 70%).
Conclusions: In the STARMEN cohort, a multivariable model that included 24-h proteinuria, serum creatinine and immunological response status at 3 months after initiation of IST predicted clinical remission at 2 years with significantly better predictive performance than baseline data or each clinical marker assessed separately.
Keywords: anti-PLA2R antibodies; clinical remission; nephrotic syndrome; predictive models; primary membranous nephropathy.
© The Author(s) (2025). Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
J.E.R.-R. declares no conflicts of interest. F.C.-F. declares that he has received consulting fees form Novartis and SOBI. A.A. declares that she has received payments for lectures from Alexion, Vifor, GSK and Otsuka, and that she has participated as Advisory Board for Samsung bioepis and Otsuka. A.-E.v.d.L., A.Sevillano, A.Shabaka, V.C., L.F.Q., M.G., P.R. and G.F.-J. declare that they have no conflicts of interest. A.O. declares that he has received grants from Sanofi, and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra AstraZeneca-UAM of chronic kidney disease and electrolytes. C.R. declares that she has received payments as speaker from Alexion, GSK and AstraZeneca. M.D. declares that she has received support for meeting/travels from Otsuka and Alexion. J.W. declares that he has received grants from Alexion, HiBio and Novartis; he also received royalties as author from UptoDate, and consulting fees from Alexion, HiBio and Otsuka. M.P. declares that he has received royalties as author from UptoDate, and payments as speaker from Novartis, Alexion, GSK and Otsuka; aditionally, he has participated as advisory board to Novartis, Samsung and Sanofi.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

