Study of Testosterone and Recombinant Human Growth Hormone in Facioscapulohumeral Muscular Dystrophy
- PMID: 40900971
- PMCID: PMC12401552
- DOI: 10.1212/NXG.0000000000200292
Study of Testosterone and Recombinant Human Growth Hormone in Facioscapulohumeral Muscular Dystrophy
Abstract
Background and objectives: Effective therapies for facioscapulohumeral muscular dystrophy (FSHD) are currently limited. Recombinant human growth hormone (rHGH) combined with testosterone (combination therapy) may have meaningful clinical effects on ambulation, strength, muscle mass, and disease burden. As such, combination therapy has the potential to limit disease progression and functional decline in individuals with muscular dystrophy. The objective of this study was to evaluate the safety, tolerability, and potential efficacy of combination therapy in adult men with FSHD.
Methods: This investigator-initiated, single-center (University of Rochester), single-arm, proof-of-concept study evaluated the safety and tolerability of combination therapy in ambulatory adult men with FSHD. Participants received daily rHGH combined with testosterone enanthate injections every 2 weeks for 24 weeks, followed by a 12-week washout period. Participants underwent serial safety and laboratory assessments to monitor safety and tolerability during the study. Participants were also evaluated for changes from baseline in lean body mass (LBM) and fat mass, measured by dual-energy X-ray absorptiometry; ambulation, measured by 6-minute walk distance; strength; clinical function, measured using the FSHD-Composite Outcome Measure (FSHD-COM); and patient-reported disease burden, measured by the FSHD Health Index (FSHD-HI).
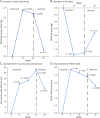
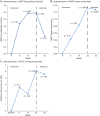
Results: Nineteen of 20 participants completed the study, with no participants experiencing a serious adverse event. The most common adverse event was mild injection site reaction at the rHGH and/or testosterone injection site. After 24 weeks, LBM improved by 2.21 kilograms (95% CI 1.35-3.07; p < 0.0001), fat mass decreased by 1.30 kilograms (95% CI -2.56 to -0.04; p = 0.04), 6-minute walk distance increased by 37.3 m (95% CI 18.3-56.9; p = 0.001), overall strength (average % of predicted normal) increased by 3% (95% CI 0.3-5.6; p = 0.03), clinical function (FSHD-COM) improved by 2.4 points (95% CI 4.0-0.8; p = 0.006), and total disease burden (FSHD-HI) decreased by 6.1 points (95% CI -12.0 to -0.2; p = 0.04).
Discussion: Combination therapy was safe and well tolerated in men with FSHD. Participants experienced improvements in ambulation, strength, muscle mass, and disease burden after receiving this study intervention. Larger randomized, double-blind, placebo-controlled trials are needed to further investigate this promising therapeutic approach.
Trial registration information: Registered on ClinicalTrials.gov: NCT03123913.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
Chad Heatwole receives royalties for the use of multiple disease-specific instruments. He has provided consultation to Biogen Idec, Ionis Pharmaceuticals, aTyr Pharma, AMO Pharma, Acceleron Pharma, Cytokinetics, Expansion Therapeutics, Harmony Biosciences, Regeneron Pharmaceuticals, Astellas Pharmaceuticals, AveXis, Recursion Pharmaceuticals, Iris Medicine, Inc., Takeda Pharmaceutical Company, Scholar Rock, Avidity Biosciences, Novartis Pharmaceuticals Corporation, SwanBio Therapeutics, Neurocrine Biosciences, Sanofi, Lupin Pharmaceuticals, Vertex Pharmaceuticals, Dyne Therapeutics, Applied Therapeutics, and the Marigold Foundation. He receives grant support from the Department of Defense, Duchenne UK, Parent Project Muscular Dystrophy, Recursion Pharmaceuticals, Swan Bio Therapeutics, Sanofi, Lupin Pharmaceuticals, the NINDS, the Muscular Dystrophy Association, the Friedreich's Ataxia Research Alliance, Cure Spinal Muscular Atrophy, the Amyotrophic Lateral Sclerosis Association, the University of Miami, Novartis Pharmaceuticals Corporation, and the M.J. Foxx Foundation. He is the director of the University of Rochester's Center for Health + Technology. E. Luebbe has no disclosures. J. Hamel consults for Vertex Pharmaceuticals. P. Mongiovi has no disclosures. E. Ciafaloni has received personal compensation for serving on advisory boards, on DMCs, and/or as a consultant for Alexion, Argenx, Biogen, Momenta, Pfizer, Sarepta Therapeutics, Janssen, Italfarmaco, NS Pharma, Regenxbio, and ML Bio Solutions. N. Dilek, W. Martens, D. Weber, H. Rashid, J. Allen, C. Smith, S. Howell, and S. Rosero report no disclosures. K. Eichinger has received personal compensation for serving on advisory boards and/or as a consultant for Fulcrum Therapeutics, Avidity Biosciences, Roche, Dyne Therapeutics, and TRiNDS. She has received royalties for the FSHD-COM. L. Baker has no disclosures. J. Dekdebrun consults for Avidity Biosciences, Arthex, Dyne Therapeutics, Lupin, Pepgen, Vertex Pharmaceuticals, TRiNDS, and Atom. J. Hilbert and A. Varma report no disclosures. C. Thornton provides consulting to Biogen, Vertex Pharmaceuticals, Entrada Therapeutics, and Avidity Biosciences; received honoraria from Sanofi; served on Scientific Advisory Boards for Dyne Therapeutics and PepGen; and serves on the Board for the Myotonic Dystrophy Foundation. M.P. McDermott receives grant funding from the NIH, FDA, and Cure SMA and has served on Data and Safety Monitoring Boards for NIH, Eli Lilly and Company, Neurocrine Biosciences, Inc., ReveraGen BioPharma, Inc., NS Pharma, Inc., Prilenia Therapeutics Development, Ltd., and Seelos Therapeutics, Inc. R.T. Moxley has no disclosures. Go to Neurology.org/NG for full disclosures.TAKE-HOME POINTS→ This study investigates the safety, tolerability, and preliminary efficacy of testosterone and rHGH combination therapy in adult men with facioscapulohumeral muscular dystroph (FSHD).→ In this proof-of-concept clinical trial of 20 participants with FSHD, the combination therapy was well tolerated with no serious adverse events. After 24 weeks, lean body mass (LBM), 6-minute walk distance, overall strength, clinical function, and disease burden improved from baseline.→ The use of combination therapy for FSHD to recover muscle mass, ambulation, and function holds promise and warrants further study through a larger, randomized controlled trial.
Figures

References
-
- Padberg GWaM. Facioscapulohumeral Disease; 1982. Accessed Feb 27, 2023. hdl.handle.net/1887/25818
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
