EPEC autotransporter adhesin (Eaa): a novel adhesin identified in atypical enteropathogenic Escherichia coli
- PMID: 40900999
- PMCID: PMC12399667
- DOI: 10.3389/fcimb.2025.1617101
EPEC autotransporter adhesin (Eaa): a novel adhesin identified in atypical enteropathogenic Escherichia coli
Abstract
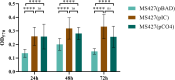
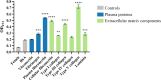
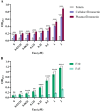
Enteropathogenic Escherichia coli (EPEC) is a pathogen that causes diarrhea that can be subdivided into typical (tEPEC) and atypical (aEPEC), based on the production of an adhesin termed Bundle-Forming Pilus (BFP) in the former group. aEPEC is one of the main bacterial pathogens isolated from individuals with diarrhea, and some serotypes have been implicated in diarrheal outbreaks in Brazil, such as the O2:H16. A comparative genomic analysis of aEPEC of this serotype led to the identification of a gene encoding a previously uncharacterized autotransporter protein. In the present study, this novel autotransporter protein was characterized and named EPEC Autotransporter Adhesin (Eaa). The Eaa-encoding gene (eaa) is located in a chromosomal prophage region of 17,014 base pairs, organized in 20 open reading frames and inserted downstream to the threonine-tRNA. A recombinant plasmid termed pIC (pBAD/Myc-His A harboring the eaa gene from aEPEC BA92) was transformed in the MS427 host bacteria, and the MS427(pIC) was used in phenotypic assays. Immunogold-labelling transmission electron microscopy, using anti-Eaa antibodies, showed the presence of Eaa in the cell surface of the wild-type BA92 and MS427(pIC) strains. Subsequently, we demonstrated that Eaa mediates bacterial autoaggregation, biofilm formation and binding to several components of the extracellular matrix, including fibrinogen, plasma and cellular fibronectin, type I, III as well as V collagen and laminin. In summary, we demonstrated that Eaa harbors several adherence properties and may contribute to the pathogenicity of some aEPEC isolates by mediating the interaction of this pathogen with biotic and abiotic surfaces.
Keywords: adhesin; atypical EPEC; autotransporter protein; bacterial pathogenesis; biofilm; fibronectin.
Copyright © 2025 Orsi, de Lira, Castilho, de Souza, Onur, Chura-Chambi, Abe, Carvalho, dos Santos, Rasko, Schembri, Barbosa, Elias and Hernandes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abe C. M., Salvador F. A., Falsetti I. N., Vieira M. A. M., Blanco J., Blanco J. E., et al. (2008). Uropathogenic Escherichia coli (UPEC) strains may carry virulence properties of diarrhoeagenic E. coli. FEMS Immunol. Med. Microbiol. 52, 397–406. doi: 10.1111/j.1574-695X.2008.00388.x, PMID: - DOI - PubMed
-
- Abe C. M., Trabulsi L. R., Blanco J., Blanco M., Dhabi G., Blanco J. E., et al. (2009). Virulence features of atypical enteropathogenic Escherichia coli identified by the eae(+) EAF-negative stx(-) genetic profile. Diagn. Microbiol. Infect. Dis. 64, 357–365. doi: 10.1016/j.diagmicrobio.2009.03.025, PMID: - DOI - PubMed
-
- Abreu A. G., Bueris V., Porangaba T. M., Sircili M. P., Navarro-Garcia F., Elias W. P. (2013). Autotransporter protein-encoding genes of diarrheagenic Escherichia coli are found in both typical and atypical enteropathogenic E. coli strains. Appl. Environ. Microbiol. 79, 411–414. doi: 10.1128/AEM.02635-12, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

