Cyclodextrin-assisted photostabilization of 5-fluorouracil: a combined kinetic and computational investigation
- PMID: 40901450
- PMCID: PMC12400308
- DOI: 10.1039/d5ra05287d
Cyclodextrin-assisted photostabilization of 5-fluorouracil: a combined kinetic and computational investigation
Abstract
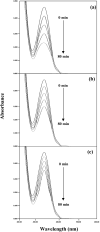
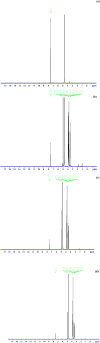
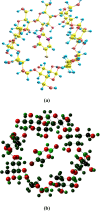
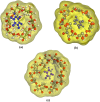
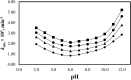
The photostabilization of 5-fluorouracil (5-FU) has been carried out in the pH range of 2.0-12.0 using cyclodextrins (α-, β-, γ-) as a complexing agent. The inclusion complex formation between 5-FU and CDs has been evaluated using conductometry, FTIR spectroscopy, NMR spectroscopy, differential scanning calorimetry (DSC), and molecular docking simulations. The loss of absorbance at 266 nm for 5-FU in the presence of CDs indicates its photodegradation. The entrapment of 5-FU in α-, β-, and γ-CDs ranges from 22.0-58.0, 30.0-64.0, and 42.0-77.0%, respectively, indicating the formation of inclusion complexes of CDs with 5-FU. The values of Stern-Volmer fluorescence quenching constants and binding constants for α-, β- and γ-CDs are 1.10, 1.69, 3.06 × 103 L mol-1 and 2.89, 3.11, 3.62 103 L mol-1, respectively. The apparent first-order rate constants (k obs) for the photodegradation of 5-FU in the presence of α-, β-, and γ-CDs are 1.75-5.12, 1.21-4.89, and 0.85-4.69 × 103, min-1, respectively. The photochemical interaction (k 2) of 5-FU with α-, β-, and γ-CDs ranges from 0.25-0.49, 0.63-1.47, and 0.79-2.14 M-1 min-1 in the pH range 2.0-12.0. The mode of photochemical interaction of 5-FU and CDs and the photostabilization of 5-FU is described based on H-atom abstraction by 5-FU from CD and radical-radical recombination. The computational analysis complemented the experimental findings, showing a significant second-order donor-acceptor interaction, particularly LP → π*, between the hydroxyl groups of γ-CD and the carbonyl groups of 5-FU. The natural population analysis further revealed a shift in electron density from γ-CD to 5-FU, indicating a protective charge-transfer mechanism that contributes to the photostabilization of 5-FU.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
The Binding Mechanism Between Cyclodextrins and Anticancer Drug Noscapine: A Spectroscopic and Molecular Docking Study.J Fluoresc. 2025 Jun;35(6):4637-4651. doi: 10.1007/s10895-024-03869-5. Epub 2024 Jul 26. J Fluoresc. 2025. PMID: 39060827
-
Cyclodextrin-mediated Enhancement of Haloperidol Solubility: Physicochemical Studies and In Vivo Investigation Using Planaria Worms.Pharm Res. 2025 Aug;42(8):1373-1383. doi: 10.1007/s11095-025-03909-0. Epub 2025 Aug 15. Pharm Res. 2025. PMID: 40813930 Free PMC article.
-
Extraction and comparison of the interactions of tetracycline antibiotics by different types of cyclodextrins using molecular docking studies.Sci Rep. 2025 Jul 21;15(1):26431. doi: 10.1038/s41598-025-04120-2. Sci Rep. 2025. PMID: 40691681 Free PMC article.
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
References
-
- British Pharmacopoeia, Monograph on 5-Fluorouracil, Her Majesty’s Stationary Office, London, UK, 2024
-
- Brayfield A., Martindale, The Complete Drug Reference, 38th edn, Pharmaceutical Press, London, 2014, pp. 795–798
-
- Rich T. A. Shepard R. C. Mosley S. T. J. Clin. Oncol. 2004;22:2214–2232. - PubMed
-
- The Merck Index, ed. M. J. O’ Neil, 15th edn, The Royal Society of Chemistry, Cambridge, UK, 2013
-
- Connors K. A., 5-Fluorouracil monograph, in Chemical Stability of Pharmaceuticals, ed. K. Connors, G. L. Amidon and V. J. Stella, 2nd edn, John Wiley, New Jersey, USA, 1986, pp. 468–473
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

