Antibacterial activity and mechanistic insights of gallium-based nanoparticles: an emerging frontier in metal-based antimicrobials
- PMID: 40901455
- PMCID: PMC12400149
- DOI: 10.1039/d5ra04216j
Antibacterial activity and mechanistic insights of gallium-based nanoparticles: an emerging frontier in metal-based antimicrobials
Abstract
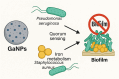
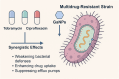
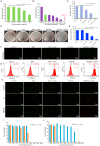
The global rise of antimicrobial resistance has intensified the search for novel therapeutic agents that act through non-conventional mechanisms. Gallium-based nanoparticles (GaNPs) represent a promising yet underexplored class of metal-based antimicrobials. Owing to their unique ability to mimic iron(iii), GaNPs disrupt key bacterial metabolic processes, particularly those dependent on iron acquisition and utilization. This mini-review provides an overview of recent advances in the development and application of GaNPs for antibacterial therapy. Emphasis is placed on their mechanisms of action, spectrum of activity, and potential biomedical applications. The review also discusses emerging insights into bacterial responses to gallium, including resistance dynamics and synergy with existing antibiotics. As an innovative approach to combat multidrug-resistant pathogens, GaNPs offer a compelling alternative to traditional antimicrobials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Osazee F. O. Mokobia K. E. et al., Addressing the Critical Role of Tungsten-Based Nanoparticles in Antibacterial Therapy. Biomed. Mater. Devices. 2024;2:614–629. doi: 10.1007/s44174-023-00127-3. - DOI
-
- Maliki M. and Omorogbe S. O.et al., Comprehensive Review of Magnetic Iron Oxide Nanoparticles for Bacterial Infection Therapy, in TMS 2023 152nd Annual Meeting & Exhibition Supplemental Proceedings, the Minerals, Metals & Materials Series, Springer, Cham, 2023, 10.1007/978-3-031-22524-6_44 - DOI
-
- Efunnuga A. Efunnuga A. Onivefu A. P. et al., Breakthroughs in Nanomedicine: The Transformative Potential of Vanadium Oxide Nanoparticles in Antimicrobial and Anticancer Treatments. BioNanoScience. 2024;14:3715–3756. doi: 10.1007/s12668-024-01566-y. - DOI
-
- Ikhuoria E. U. Uwidia I. E. Okojie R. O. et al., Synergistic Antibacterial Effects of Ternary Iron–Silver–Vanadium Oxide Nanoparticles Synthesized via Green Methods Using Customized Plant Extract Blends. Biomed. Mater. Devices. 2024;2:1186–1204. doi: 10.1007/s44174-024-00162-8. - DOI
-
- Uwidia I. E. Ikhuoria E. U. Okojie R. O. et al., Antibacterial Efficacy of Rod-Shaped Vanadium Oxide Nanostructures Synthesized Using Ganoderma lucidum Extract. Chem. Afr. 2024;7:1951–1961. doi: 10.1007/s42250-023-00854-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

