Tailoring hydrogen storage performance of Mg-Mg2Ni alloys: synergistic effects of composition and phase formation with first-principles insights
- PMID: 40901461
- PMCID: PMC12400306
- DOI: 10.1039/d5ra04356e
Tailoring hydrogen storage performance of Mg-Mg2Ni alloys: synergistic effects of composition and phase formation with first-principles insights
Abstract
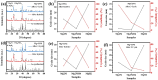
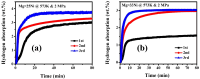
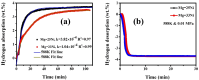
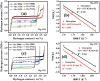
This work investigates the impact of Mg/Ni atomic ratios (75 : 25 and 66.7 : 33.3) on the formation of Mg2Ni phases and their hydrogen storage performance. Mg-Ni alloys were synthesized by vacuum casting at 1073 K followed by high-energy ball milling and were evaluated through both experimental methods and density functional theory (DFT) simulations. DFT calculations revealed that hydrogen absorption in Mg2Ni is thermodynamically more favorable than in pure Mg, with enthalpy values consistent with experimental results. Hydrogenation tests at 588 K under 20 MPa demonstrated superior performance for the Mg-25Ni alloy, which achieved a higher storage capacity (3.76 wt%) and faster kinetics than Mg-33Ni (3.53 wt%). The improved performance is attributed to the enhanced formation of MgH2 and the synergistic interaction between Mg and Mg2Ni. Kinetic analysis using the Johnson-Mehl-Avrami-Kolmogorov (JMAK) model indicated a lower activation energy for Mg-25Ni (56.74 kJ mol-1), confirming faster desorption kinetics. Pressure-composition-temperature (PCT) isotherms and van't Hoff analysis further supported the favorable thermodynamics of Mg-25Ni. Notably, this alloy exhibited excellent cyclic stability with minimal capacity loss over 10 cycles. These findings establish Mg-25Ni as a promising candidate for high-efficiency, reversible hydrogen storage, bridging fundamental insights with practical material design.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Yadav Y. K. Shaz M. A. Yadav T. P. Int. J. Hydrogen Energy. 2025;137:1137–1147. doi: 10.1016/j.ijhydene.2024.08.095. - DOI
-
- Qureshi T. Khan M. M. Pali H. S. J. Alloys Compd. 2024;1004:175668. doi: 10.1016/j.jallcom.2024.175668. - DOI
-
- Guo F. Zhang T. Shi L. Song L. J. Magnesium Alloys. 2023;11:1180–1192. doi: 10.1016/j.jma.2021.06.013. - DOI
-
- Chen Y. Zheng D. Zhou L. Cai X. Int. J. Hydrogen Energy. 2024;95:888–900. doi: 10.1016/j.ijhydene.2024.11.180. - DOI
-
- Fadonougbo J. O. Kim H.-J. Suh B.-C. Suh J.-Y. Lee Y.-S. Shim J.-H. Yim C. D. Cho Y. W. Int. J. Hydrogen Energy. 2020;45:29009–29022. doi: 10.1016/j.ijhydene.2020.07.181. - DOI
LinkOut - more resources
Full Text Sources

