Novel multimodal mechanical stimulation is superior to TENS to treat and prevent chronic low back pain: a randomized controlled trial
- PMID: 40901560
- PMCID: PMC12399623
- DOI: 10.3389/fpain.2025.1625420
Novel multimodal mechanical stimulation is superior to TENS to treat and prevent chronic low back pain: a randomized controlled trial
Abstract
Background: Low back pain (LBP) is the leading cause of disability worldwide. Up to half of moderate-to-severe acute LBP (aLBP) progress to chronic (cLBP), with neuromotor, fascial, and muscle pathology contributing to inoperable mechanical disability. A novel thermomechanical stimulation (M-Stim) device delivering stochastic and targeted vibration frequencies relieved LBP in a pilot. Efficacy versus an active control, for cLBP prevention, or reversing disability was undetermined.
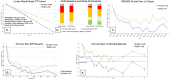
Methods: As part of a National Institutes of Health (NIH) double-blind, randomized controlled trial, 159 chiropractic patients with non-radiating moderate-to-severe LBP [Numeric Rating Scale (NRS) ≥4] were randomized to add either the multimodal M-Stim device or 4-lead transcutaneous electrical nerve stimulation (TENS) for 30 minutes daily to other therapies. Between June 2022 and July 2024, pain scores, analgesic use, and device adherence were recorded for 28 days, with weekly follow-up up to 6 months. Primary outcomes included PROMIS Pain Interference scores, NRS pain scores, and transition from aLBP to cLBP (Pain Interference ≥55 at 3 months). Exploratory analyses examined higher-severity subgroups, including those meeting NIH Research Task Force (RTF) criteria, obesity, longer pain duration, and an integrated analysis with common criteria for intractable inoperable mechanical cLBP.
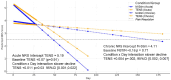
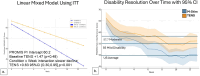
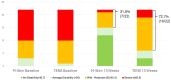
Results: For 44 aLBP and 115 cLBP participants [mean age 42.6, 54% female, BMI 30.9 (SD 6.19), NRS 5.51 (SD 2.15)], M-Stim was noninferior to TENS for initial and 10-day relief. Over time, Linear Mixed Models (intention-to-treat) showed M-Stim significantly improved pain and disability for both aLBP and cLBP, (p < .001 to p = .024). With higher severity, 23.9% (11/46) M-Stim users reached "no disability" (PROMIS = 40.7) vs. 7.1% (2/28) TENS users [RR 0.81 (95% CI 0.66-0.99), p = 0.04]. M-Stim yielded significantly greater improvement than TENS in those with pain ≥5 years, BMI ≥30, or mechanical cLBP (all p < .05). Significantly fewer aLBP M-Stim users transitioned to cLBP at 3 months [31.8% vs. 72.7%, RR 0.44 (95% CI 0.23-0.85), NNT = 2.4, p = 0.015].
Conclusions: A multimodal M-Stim device reduced progression to cLBP significantly more than TENS. Both devices reduced pain initially, but M-Stim reduced pain and disability significantly more over time, particularly in cLBP subsets with higher severity, duration, or BMI.
Clinical trial registration: https://clinicaltrials.gov/study/NCT04494698, identifier NCT04494698.
Keywords: PROMIS (patient-reported outcomes measurement information system); acute low back pain (aLBP); chronic low back pain (cLBP); focal mechanical vibration; mechanical low back pain; prevention; spine biomechanics; vibration.
© 2025 Baxter, Etnoyer-Slaski, Tucker, Williams, Swartout, Cohen and Lawson.
Conflict of interest statement
AB was employed by Harmonic Scientific LLC (parent company MMJ Labs LLC). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Delitto A, Patterson CG, Stevans JM, Brennan GP, Wegener ST, Morrisette DC, et al. PCORI Final Research Reports. Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—the TARGET Trial. Washington, DC: Patient-Centered Outcomes Research Institute (PCORI), University of Pittsburgh; (2021). Copyright © 2021. All Rights Reserved. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

