3D Genome Engineering: Current Advances and Therapeutic Opportunities in Human Diseases
- PMID: 40901634
- PMCID: PMC12399806
- DOI: 10.34133/research.0865
3D Genome Engineering: Current Advances and Therapeutic Opportunities in Human Diseases
Abstract
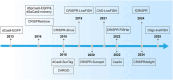
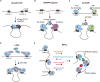
Dynamic chromatin 3-dimensional (3D) conformation is a key mechanism regulating gene expression and cellular function during development and disease. Elucidating the structure, functional dynamics, and spatiotemporal organization of the 3D genome requires integrating multiple experimental approaches, including chromatin conformation capture techniques, precise genome manipulation tools, and advanced imaging technologies. Notably, CRISPR/Cas systems have emerged as a revolutionary genome-editing platform, offering unprecedented opportunities for manipulating 3D genome organization and investigating disease mechanisms. This review systematically examines recent advances in CRISPR-based mammalian 3D genome engineering and explores the therapeutic potential of 3D genome engineering strategies in disease intervention.
Copyright © 2025 Xing Jiang et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Lanctôt C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: Regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8(2):104–115. - PubMed
-
- Zheng H, Xie W. The role of 3D genome organization in development and cell differentiation. Nat Rev Mol Cell Biol. 2019;20(9):535–550. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

