Visualizing energy transfer between redox-active colloids
- PMID: 40901935
- PMCID: PMC12407080
- DOI: 10.1126/sciadv.ady7716
Visualizing energy transfer between redox-active colloids
Abstract
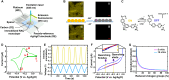
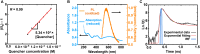
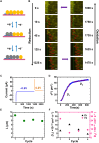
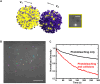
Redox-active colloids (RACs) represent a novel class of energy carriers that exchange electrical energy upon contact. Understanding contact-mediated electron transfer dynamics in RACs offers insights into physical contact events in colloidal suspensions and enables quantification of electrical energy transport in nonconjugated polymers. Redox-based electron transport was directly observed in monolayers of micron-sized RACs containing ethyl-viologen side groups via fluorescence microscopy through an unexpected nonlinear electrofluorochromism that is quantitatively coupled to the redox state of the colloid. Via imaging studies, using this electrofluorochromism, the apparent charge transfer diffusion coefficient DCT of the RAC was easily determined. The visualization of energy transport within suspensions of redox-active colloids was also demonstrated. Our work elucidates fundamental mechanisms of energy transport in colloidal systems, informs the development of next-generation redox flow batteries, and may inspire new designs of smart active soft matter including conductive polymers for applications ranging from electrochemical sensors and organic electronics to colloidal robotics.
Figures
References
-
- Berggren M., Malliaras G. G., How conducting polymer electrodes operate. Science 364, 233–234 (2019). - PubMed
-
- Luo L., Choi S. H., Frisbie C. D., Probing hopping conduction in conjugated molecular wires connected to metal electrodes. Chem. Mater. 23, 631–645 (2011).
-
- M. E. Lyons, Electroactive Polymer Electrochemistry: Part 1: Fundamentals (Springer, 2013).
-
- Baradwaj A. G., Wong S. H., Laster J. S., Wingate A. J., Hay M. E., Boudouris B. W., Impact of the addition of redox-active salts on the charge transport ability of radical polymer thin films. Macromolecules 49, 4784–4791 (2016).
-
- Tomlinson E. P., Hay M. E., Boudouris B. W., Radical polymers and their application to organic electronic devices. Macromolecules 47, 6145–6158 (2014).
LinkOut - more resources
Full Text Sources
Miscellaneous

