Randomized Phase II Trial of Pazopanib Versus Placebo in Patients With Advanced Extrapancreatic Neuroendocrine Tumors (Alliance A021202)
- PMID: 40902132
- PMCID: PMC12457709
- DOI: 10.1200/JCO-24-02644
Randomized Phase II Trial of Pazopanib Versus Placebo in Patients With Advanced Extrapancreatic Neuroendocrine Tumors (Alliance A021202)
Abstract
Purpose: Patients with advanced, well-differentiated extrapancreatic neuroendocrine tumors (epNETs) have limited systemic treatment options. Pazopanib, an oral multikinase inhibitor with activity against vascular endothelial growth factor receptor (VEGFR)-2 and -3, PDGFR-alpha and-beta, and c-Kit, was tested for efficacy in epNET.
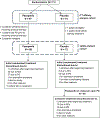
Patients and methods: We conducted a multicenter, randomized, double-blind, phase II study of pazopanib (800 mg once daily) versus placebo in low- to intermediate-grade epNET with radiologic progressive disease (PD) within 12 months of study entry. Previous somatostatin analog (SSA) was required for midgut tumors, and concurrent SSA was allowed. The primary end point was progression-free survival (PFS) by blinded independent central review. Unblinding and crossover were allowed if PD was confirmed by central review.
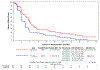
Results: One hundred seventy-one patients (97 pazopanib and 74 placebo) were randomly assigned between September 2013 and October 2015. The majority had a midgut primary site (75%) and previous SSA treatment (93%). About half (49%) of the patients had functional tumors. The median follow-up was 61 months (95% CI, 60 to 63). Median PFS was 11.8 versus 7.6 months in pazopanib versus placebo, respectively (hazard ratio, 0.54 [95% CI, 0.37 to 0.79]; P < .001); 49 placebo patients crossed over to pazopanib. There was no significant difference in overall survival between the treatment arms. Rates of grade 3 or greater adverse events (regardless of attribution) were higher in pazopanib versus placebo (84% v 47%; P < .001), as were grade 5 death events (8% v 0%, P = .017).
Conclusion: Pazopanib compared with placebo significantly improves PFS in patients with progressive epNET, confirming that the VEGF signaling pathway is a valid target for therapy in epNET. However, after integrating the associated risks relative to the benefits, further development of pazopanib in this clinical context is not planned.
Trial registration: ClinicalTrials.gov NCT01841736.
Figures



References
-
- Sedlack AJH, Varghese DG, Naimian A, et al. : Update in the management of gastroenteropancreatic neuroendocrine tumors. Cancer 130:3090–3105, 2024 - PubMed
-
- Caplin M, Pavel M, Cwikla J, et al. : Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:224–33, 2014 - PubMed
-
- Rinke A, Müller H-H, Schade-Brittinger C, et al. : Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. Journal of Clinical Oncology 27:4656–4663, 2009 - PubMed
-
- Vinik AI, Wolin EM, Liyanage N, et al. : Evaluation of Lanreotide Depot/Autogel Efficacy and Safety as a Carcinoid Syndrome Treatment (Elect): A Randomized, Double-Blind, Placebo-Controlled Trial. Endocr Pract 22:1068–80, 2016 - PubMed
-
- Rubin J, Ajani J, Schirmer W, et al. : Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol 17:600–6, 1999 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

