Xevinapant or Placebo Plus Platinum-Based Chemoradiotherapy in Unresected Locally Advanced Squamous Cell Carcinoma of the Head and Neck (TrilynX): A Randomized, Phase III Study
- PMID: 40902136
- PMCID: PMC12509437
- DOI: 10.1200/JCO-25-00272
Xevinapant or Placebo Plus Platinum-Based Chemoradiotherapy in Unresected Locally Advanced Squamous Cell Carcinoma of the Head and Neck (TrilynX): A Randomized, Phase III Study
Abstract
Purpose: TrilynX was a randomized, double-blind, phase III study evaluating the addition of xevinapant (an inhibitor of apoptosis proteins inhibitor) or placebo to chemoradiotherapy (CRT) in patients with unresected locally advanced squamous cell carcinoma of the head and neck (LA SCCHN).
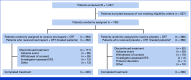
Methods: Patients with unresected LA SCCHN (oropharynx [p16-negative only], hypopharynx, or larynx) were randomly assigned 1:1 to six cycles of oral xevinapant 200 mg/day or matched placebo (once daily on Days 1-14 of a 21-day cycle) plus CRT for the first three cycles (cisplatin [100 mg/m2 once on Day 2 of every cycle] plus intensity-modulated radiotherapy [70 Gy; 35 fractions of 2 Gy/day, 5 days/week]). The primary end point was event-free survival (EFS) assessed by the blinded independent review committee. Progression-free survival, overall survival (OS), and safety were secondary end points.
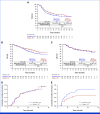
Results: Between September 20, 2020, and February 27, 2023, 730 patients were randomly assigned to xevinapant plus CRT (n = 364) or placebo plus CRT (n = 366). The median (95% CI) EFS was 19.4 months (14.5 to not estimable) with xevinapant and 33.1 months (21.0 to not estimable) with placebo (hazard ratio [HR], 1.33 [95% CI, 1.05 to 1.67]; P = .9919). OS was worse in the xevinapant arm (HR, 1.39 [95% CI, 1.04 to 1.86]). Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 320 (87.9%; xevinapant) and 286 (80.3%; placebo) patients; anemia (78 [21.4%] v 51 [14.3%]) and neutropenia (71 [19.5%] v 69 [19.4%]) were the most common. Serious TEAEs occurred in 194 (53.3%; xevinapant) and 129 (36.2%; placebo) patients. TEAEs leading to death occurred in 22 (6.0%; xevinapant) and 13 (3.7%; placebo) patients.
Conclusion: Xevinapant plus CRT did not improve EFS (EFS was shorter with xevinapant v placebo) and demonstrated an unfavorable safety profile versus placebo plus CRT in patients with unresected LA SCCHN.
Trial registration: ClinicalTrials.gov NCT04459715.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Psyrri A, Rampias T, Vermorken JB. The current and future impact of human papillomavirus on treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:2101–2115. - PubMed
-
- NCCN Clinical Practice Guidelines in Oncology Head and neck cancer. v5. 2024
-
- Ang KK. Multidisciplinary management of locally advanced SCCHN: Optimizing treatment outcomes. Oncologist. 2008;13:899–910. - PubMed
-
- Machiels JP, René Leemans C, Golusinski W, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1462–1475. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

