Denosumab versus zoledronic acid in metastatic breast cancer: A retrospective observational analysis of 24-dose regimens on analgesia and skeletal-related event prevention
- PMID: 40902303
- PMCID: PMC12444453
- DOI: 10.1016/j.breast.2025.104565
Denosumab versus zoledronic acid in metastatic breast cancer: A retrospective observational analysis of 24-dose regimens on analgesia and skeletal-related event prevention
Abstract
Background: Based on available data in the literature, current evidence supporting the analgesic role of antiresorptive drugs is weak. This study compared the efficacy of zoledronic acid (ZA) and denosumab (Dmab) in reducing bone pain and first Skeletal Related Events (SREs) in real world setting.
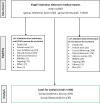
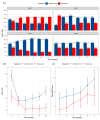
Methods: A retrospective observational cohort study was conducted in patients with female breast cancer-related bone metastases at the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan, Italy, from January 2008 to January 2023. Patients were included if they had undergone at least 24 consecutive administrations of ZA or Dmab. The primary endpoint was the analgesic effect, evaluated in terms of average pain intensity, analgesic drug use (Word Health Organization analgesic ladder), and daily opioid doses (oral morphine equivalent, OME) assessed at 3, 6, 12, 18, and 24 months, analyzed by Bayesian longitudinal mixed-effects models. Secondary endpoints included first SREs and radiotherapy/surgery incidence.
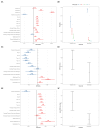
Results: Among 364 patients (194 ZA, 170 Dmab), Dmab demonstrated a significant analgesic advantage. Dmab group showed an 89 % lower likelihood of increasing one analgesic ladder step, a mean reduction of 0.4 points on the numerical rating scale (95 % CI, -0.7, -0.1), and lower daily OME doses (0.77 mg vs. 6.2 mg for ZA). At 12 and 24 months, ZA and Dmab showed similar cumulative incidences of SREs and radiotherapy/surgery (p = 0.601 and p = 0.923).
Conclusions: Dmab showed consistent superior effect than ZA in reducing bone pain in metastatic BC, yet both treatments delivered similar protection against SREs and the need for radiotherapy or surgery.
Keywords: Bone metastases; Bone pain; Breast cancer; Denosumab; Skeletal-related events; Zoledronic acid.
Copyright © 2025. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Giacomo Massa reports a relationship with Leopharma, Merck that includes: board membership and travel reimbursement.Dr Zecca has received honoraria from Amgen.Prof Caraceni has received honoraria from Angelini, Shionogi, Kyowa Kirin, Molteni, Pfizer/Eli Lilly Italia SPA.Dr Bianchi has received honoraria from Roche, Novartis, Seagen, AstraZeneca/Daiichi Sankyo, Lilly.All remaining authors have declared no conflict of interest.
Figures

References
-
- Cetin K., Christiansen C.F., Sværke C., Jacobsen J.B., Sørensen H.T. Survival in patients with breast cancer with bone metastasis: a Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open. 2015 Apr 29;5(4) doi: 10.1136/bmjopen-2015-007702. - DOI - PMC - PubMed
-
- Coleman R., Cancer Network Impact of bone-targeted treatments on skeletal morbidity and survival in breast cancer. 2016. https://www.cancernetwork.com/view/impact-bone-targeted-treatments-skele... [cited 2024 Dec 23] - PubMed

