Feature-driven whole-tissue imaging with subcellular resolution
- PMID: 40902591
- PMCID: PMC12539259
- DOI: 10.1016/j.crmeth.2025.101148
Feature-driven whole-tissue imaging with subcellular resolution
Abstract
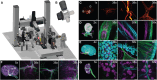
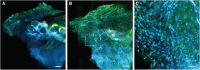
Existing microscopy approaches are often unable to identify and contextualize rare but biologically meaningful events due to limitations associated with simultaneously achieving both high-resolution imaging and a cm-scale field of view. Here, we present multiscale cleared tissue axially swept light-sheet microscopy (MCT-ASLM), a platform combining cm-scale imaging with targeted high-resolution interrogation of intact tissues in human-guided or autonomous modes. Capable of capturing fields of view up to 21 mm at micron-scale resolution, MCT-ASLM can seamlessly transition to a targeted imaging mode with an isotropic resolution that approaches ∼300 nm. This versatility enables detailed studies of hierarchical organization and spatially complex processes, including mapping neuronal circuits in rat brains, visualizing glomerular innervation in mouse kidneys, and examining metastatic tumor microenvironments. By bridging subcellular- to tissue-level scales, MCT-ASLM offers a powerful method for unraveling how local events contribute to global biological phenomena.
Keywords: CP: Imaging; LSFM; cancer; development; feature-driven cleared tissue imaging; multiscale ASLM; neuroscience.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.M.D. is a founder of Discovery Imaging Systems, LLC, has a patent covering ASLM, and has consultancy agreements with 3i, Inc (Denver, CO, USA).
Figures




Update of
-
Feature-Driven Whole-Tissue Imaging with Subcellular Resolution.bioRxiv [Preprint]. 2025 Jan 26:2025.01.23.634600. doi: 10.1101/2025.01.23.634600. bioRxiv. 2025. Update in: Cell Rep Methods. 2025 Sep 15;5(9):101148. doi: 10.1016/j.crmeth.2025.101148. PMID: 39896507 Free PMC article. Updated. Preprint.
References
-
- Stelzer E.H.K., Strobl F., Chang B.-J., Preusser F., Preibisch S., McDole K., Fiolka R. Light sheet fluorescence microscopy. Nat. Rev. Methods Primers. 2021;1 doi: 10.1038/s43586-021-00069-4. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

