Blood Clock Correlation Distance (BloodCCD) as a novel marker to detect circadian rhythm disruption in cancer survivors with insomnia
- PMID: 40903493
- PMCID: PMC12408818
- DOI: 10.1038/s44276-025-00176-9
Blood Clock Correlation Distance (BloodCCD) as a novel marker to detect circadian rhythm disruption in cancer survivors with insomnia
Abstract
Background: Insomnia is a toxicity of cancer and treatment for survivors without an objective biochemical measure. Circadian rhythms are 24-h cycles that influence physiologic processes including sleep, and disrupted rhythms may contribute to insomnia. Here, we use BloodCCD to assess circadian rhythms from RNA-sequencing of blood from cancer survivors with insomnia from the YOCAS-II trial. YOCAS-II aimed to determine whether YOCAS©® yoga or cognitive behavioral therapy for insomnia (CBT-I) improved insomnia in survivors compared with a behavioral placebo. We hypothesized that circadian rhythms are disrupted in survivors, and that insomnia severity correlates with the degree of circadian disruption.
Methods: BloodCCD was developed to biochemically assess circadian rhythms in blood. It was adapted from the previously published Clock Correlation Distance (CCD) and uses a correlation matrix of 42 genes known to oscillate throughout the day in blood.
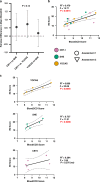
Results: Cancer survivors had higher (worse) BloodCCD scores, indicating disrupted circadian clock, compared to healthy individuals. Furthermore, insomnia severity correlated with worse BloodCCD, and those in the YOCAS and behavioral placebo arm showed significant correlation between BloodCCD score and insomnia.
Conclusions: BloodCCD shows promise as a biomarker to biochemically detect disrupted circadian rhythms in cancer survivors, and as an indicator for insomnia severity.
Clinical trial identifier: NCT02613364.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The YOCAS-II trial (NCT02613364) including the consent form, all original study procedures, and all procedures associated with this study and use of biological samples, was approved by a central Institutional Review Board (IRB) at the University of Rochester and all IRBs at collaborating sites. All participants provided informed consent which included agreement to provide biological samples to be banked and used for future research. The YOCAS-II trial was approved by the Research Subjects Review Board (RSRB) is the University’s IRB, STUDY00000554, and the BloodCCD research was deemed exempt and approved by the RSRB, STUDY00005516. This study was performed in accordance with the Declaration of Helsinki. Consent for publication: This manuscript contains no personally identifiable information (PII) or protected health information (PHI).
Figures



References
-
- Schultz PN, Klein MJ, Beck ML, Stava C, Sellin RV. Breast cancer: relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nurs. 2005;14:204–11. - PubMed
-
- Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864–6. - PubMed
-
- Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580–6. - PubMed
-
- Partinen M. Epidemiology of sleep disorders. Handb Clin Neurol. 2011;98:275–314. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
