Single-cell transcriptomic and genomic changes in the ageing human brain
- PMID: 40903571
- PMCID: PMC12527935
- DOI: 10.1038/s41586-025-09435-8
Single-cell transcriptomic and genomic changes in the ageing human brain
Abstract
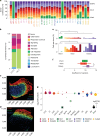
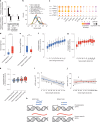
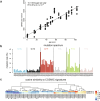
Over time, cells in the brain and in the body accumulate damage, which contributes to the ageing process1. In the human brain, the prefrontal cortex undergoes age-related changes that can affect cognitive functioning later in life2. Here, using single-nucleus RNA sequencing (snRNA-seq), single-cell whole-genome sequencing (scWGS) and spatial transcriptomics, we identify gene-expression and genomic changes in the human prefrontal cortex across lifespan, from infancy to centenarian. snRNA-seq identified infant-specific cell clusters enriched for the expression of neurodevelopmental genes, as well as an age-associated common downregulation of cell-essential homeostatic genes that function in ribosomes, transport and metabolism across cell types. Conversely, the expression of neuron-specific genes generally remains stable throughout life. These findings were validated with spatial transcriptomics. scWGS identified two age-associated mutational signatures that correlate with gene transcription and gene repression, respectively, and revealed gene length- and expression-level-dependent rates of somatic mutation in neurons that correlate with the transcriptomic landscape of the aged human brain. Our results provide insight into crucial aspects of human brain development and ageing, and shed light on transcriptomic and genomic dynamics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












Update of
-
Single-cell transcriptomic and genomic changes in the aging human brain.bioRxiv [Preprint]. 2023 Nov 7:2023.11.07.566050. doi: 10.1101/2023.11.07.566050. bioRxiv. 2023. Update in: Nature. 2025 Oct;646(8085):657-666. doi: 10.1038/s41586-025-09435-8. PMID: 37986960 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

