A brain-wide map of neural activity during complex behaviour
- PMID: 40903598
- PMCID: PMC12408349
- DOI: 10.1038/s41586-025-09235-0
A brain-wide map of neural activity during complex behaviour
Abstract
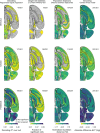
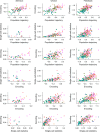
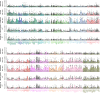
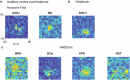
A key challenge in neuroscience is understanding how neurons in hundreds of interconnected brain regions integrate sensory inputs with previous expectations to initiate movements and make decisions1. It is difficult to meet this challenge if different laboratories apply different analyses to different recordings in different regions during different behaviours. Here we report a comprehensive set of recordings from 621,733 neurons recorded with 699 Neuropixels probes across 139 mice in 12 laboratories. The data were obtained from mice performing a decision-making task with sensory, motor and cognitive components. The probes covered 279 brain areas in the left forebrain and midbrain and the right hindbrain and cerebellum. We provide an initial appraisal of this brain-wide map and assess how neural activity encodes key task variables. Representations of visual stimuli transiently appeared in classical visual areas after stimulus onset and then spread to ramp-like activity in a collection of midbrain and hindbrain regions that also encoded choices. Neural responses correlated with impending motor action almost everywhere in the brain. Responses to reward delivery and consumption were also widespread. This publicly available dataset represents a resource for understanding how computations distributed across and within brain areas drive behaviour.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures















References
-
- Broca, P. Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole) [in French]. Bull. Mem. Soc. Anatom. de Paris6, 330–357 (1861).
-
- Lashley, K. S. Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain (Univ. Chicago Press, 1929).

