Tirzepatide Associated With Improved Health-Related Quality of Life in Adults With Obesity or Overweight in SURMOUNT-4
- PMID: 40903801
- PMCID: PMC12559773
- DOI: 10.1002/oby.70011
Tirzepatide Associated With Improved Health-Related Quality of Life in Adults With Obesity or Overweight in SURMOUNT-4
Abstract
Objective: In SURMOUNT-4, participants with obesity or overweight regained substantial weight after tirzepatide withdrawal, whereas continued treatment resulted in additional weight reduction. We evaluated the effect of continued tirzepatide treatment on health-related quality of life (HRQoL) in SURMOUNT-4.
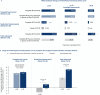
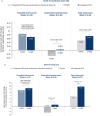
Methods: Participants who achieved tirzepatide maximum tolerated dose (MTD; 10 or 15 mg; N = 670) during a 36-week (W) lead-in period were randomized (1:1) to continue receiving tirzepatide MTD or switch to placebo through W88. HRQoL was assessed using patient-reported outcomes (PROs: SF-36v2, IWQOL-Lite-CT, EQ-5D-5L). Post hoc analysis included changes in PROs by weight reduction categories and baseline Patient Global Impression of Status for physical activity response categories among tirzepatide-treated participants and by weight regain categories in the placebo group.
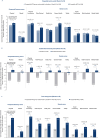
Results: Tirzepatide treatment was associated with improved PROs from W0 to W36, and these improvements were maintained from W36 to W88 with continued tirzepatide treatment versus placebo. Tirzepatide-treated participants with greater weight reduction and those with physical function limitations at baseline showed numerically greater improvements in PROs. Greater weight regain among participants who switched to placebo was associated with worsening of PROs.
Conclusions: In participants with overweight or obesity, continued tirzepatide treatment was associated with maintaining HRQoL improvement, while treatment withdrawal resulted in worsening of HRQoL.
Trial registration: ClinicalTrials.gov identifier NCT04660643.
Keywords: obesity; patient‐reported outcomes; tirzepatide health‐related quality of life.
© 2025 Eli Lilly and Company. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
Theresa Hunter Gibble, Dachuang Cao, Madhumita Murphy, Irina Jouravskaya, and Birong Liao are employees and stockholders of Eli Lilly and Company, Indianapolis, United States. Harold Edward Bays (H.E.B.)'s research site institution has received research grants from 89Bio, Allergan, Alon Medtech/Epitomee, Aligos, Altimmune, Amgen, Anji Pharma, AbbVie, AstraZeneca, Bioage, Bionime, Boehringer Ingelheim, Carmot, Chorus/Bioage, Eli Lilly, Esperion, Evidera, Fractyl, GlaxoSmithKline, HighTide, Home Access, Horizon, Ionis, Kallyope, LG‐Chem, Madrigal, Merck, Mineralys, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Satsuma, Selecta, Shionogi, Skye/Birdrock, TIMI, Veru, Viking, and Vivus. H.E.B. has served as a consultant/advisor for 89Bio, Altimmune, Amgen, Boehringer Ingelheim, Kiniksa, HighTide, Lilly, Novo Nordisk, Regeneron, Veru, Zomagen, and ZyVersa.
Figures


References
-
- “Health Risks of Overweight & Obesity,” NIDDK, https://www.niddk.nih.gov/health‐information/weight‐management/adult‐ove....
-
- Barnard‐Kelly K., Battelino T., Brosius F. C., et al., “Defining Patient‐Reported Outcomes in Diabetes, Obesity, Cardiovascular Disease, and Chronic Kidney Disease for Clinical Practice Guidelines‐Perspectives of the Taskforce of the Guideline Workshop,” Cardiovascular Diabetology 24 (2025): 68. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

