Cancer Cell-Secreted miR-33a Reduces Stress Granule Formation by Targeting Polyamine Metabolism in Stroma to Promote Tumourigenesis
- PMID: 40903826
- PMCID: PMC12408367
- DOI: 10.1002/jev2.70153
Cancer Cell-Secreted miR-33a Reduces Stress Granule Formation by Targeting Polyamine Metabolism in Stroma to Promote Tumourigenesis
Abstract
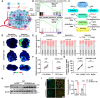
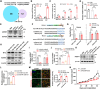
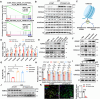
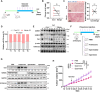
Tumour progression depends on the bidirectional interactions between cancer and stroma in the heterogeneous tumour microenvironment (TME) partially through extracellular vesicles (EVs). However, the secretary mechanism and biological effect of cancer cell derived EVs on tumour survival under starvation is poorly defined. Here, we identify cancer cells selectively secrete miR-33a with the assistance of aconitase 1 (ACO1), an iron-responsive RNA binding protein, under glucose starvation and lower iron level, which affiliates the binding capability of miR-33a and ACO1. Exosomal miR-33a suppresses putrescine biosynthesis by targeting AGMAT in cancer-associated fibroblasts (CAFs) from tumour core region, where putrescine inhibits the expression of demethylase KDM5C. TIA1 gene, stress granule (SG) marker, is tightly regulated by miR-33a/KDM5C/H3K4me3 axis and exosomal miR-33a diminishes the formation of stromal SGs in CAFs. Collectively, our study reveals tumour selectively secretes miR-33a-5p through EVs to remodel the stromal SG formation and gain survival possibility for cancer cells in tumour core region, highlighting a novel regulatory mechanism of iron and nutrient level on EV secretion and the function of polyamine metabolism in reshaping epigenetic profiles.
Keywords: epigenetic reprogramming; extracellular vesicles; polyamine metabolism; stress granules; tumour microenvironment.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Al‐Habsi, M. , Chamoto K., Matsumoto K., et al. 2022. “Spermidine Activates Mitochondrial Trifunctional Protein and Improves Antitumor Immunity in Mice.” Science 378, no. 6618: eabj3510. - PubMed
-
- Bogenhagen, D. , and Clayton D. A.. 1974. “The Number of Mitochondrial Deoxyribonucleic Acid Genomes in Mouse L and Human HeLa Cells. Quantitative Isolation of Mitochondrial Deoxyribonucleic Acid.” Journal of Biological Chemistry 249, no. 24: 7991–7995. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

