Endothelin-Converting Enzyme-Like 1 Regulated by LIF Contributes to Chronic Constriction Injury-Induced Neuropathic Pain in Mice
- PMID: 40904192
- PMCID: PMC12409069
- DOI: 10.1111/cns.70578
Endothelin-Converting Enzyme-Like 1 Regulated by LIF Contributes to Chronic Constriction Injury-Induced Neuropathic Pain in Mice
Abstract
Aims: This study is to investigate the role of Endothelin-converting enzyme-like 1 (ECEL1) in neuropathic pain (NP).
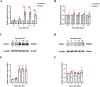
Methods: The expression of ECEL1 was modulated by injecting adeno-associated virus 5 (AAV5) carrying Ecel1 shRNA or full-length Ecel1 into the dorsal root ganglion (DRG) of mice with a chronic constriction injury (CCI) model. Then, various nociceptive responses were evaluated. Additionally, leukemia inhibitory factor (LIF) was intrathecally injected, or its function was blocked, to observe the changes in ECEL1 expression.
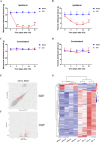
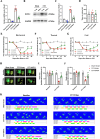
Results: Our findings demonstrate that downregulating ECEL1 expression alleviates CCI-induced pain and reduces the hyperexcitability of injured DRG neurons, which is achieved by inhibiting sympathetic sprouting in the DRG. Conversely, overexpressing ECEL1 in DRG neurons leads to pain hypersensitivity. Additionally, we observed that LIF upregulated ECEL1 expression, while blocking LIF reduced ECEL1 expression and mitigated CCI-induced nociception in mice.
Conclusion: ECEL1 promotes hyperalgesia following CCI and is regulated by LIF, suggesting it could be a new target for NP treatment.
Keywords: DRG; ECEL1; LIF; neuropathic pain; sympathetic sprouting.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
RNA-binding protein SYNCRIP contributes to neuropathic pain through stabilising CCR2 expression in primary sensory neurones.Br J Anaesth. 2024 Nov;133(5):1028-1041. doi: 10.1016/j.bja.2024.07.024. Epub 2024 Sep 7. Br J Anaesth. 2024. PMID: 39244479
-
NaHS alleviates neuropathic pain in mice by inhibiting IL-17-mediated dopamine (DA) neuron necroptosis in the VTA.Brain Res Bull. 2025 Jan;220:111168. doi: 10.1016/j.brainresbull.2024.111168. Epub 2024 Dec 11. Brain Res Bull. 2025. PMID: 39672209
-
Tuina Alleviates Neuropathic Pain in CCI Rats by Regulating the TRPV4-CaMKII Signaling Pathway in Dorsal Root Ganglion.Pain Res Manag. 2025 Aug 6;2025:3697374. doi: 10.1155/prm/3697374. eCollection 2025. Pain Res Manag. 2025. PMID: 40809812 Free PMC article.
-
Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Sep 14;9(9):CD011976. doi: 10.1002/14651858.CD011976.pub2. Cochrane Database Syst Rev. 2017. PMID: 28905362 Free PMC article.
-
Venlafaxine for neuropathic pain in adults.Cochrane Database Syst Rev. 2015 Aug 23;2015(8):CD011091. doi: 10.1002/14651858.CD011091.pub2. Cochrane Database Syst Rev. 2015. PMID: 26298465 Free PMC article.
References
-
- Finnerup N. B., Kuner R., and Jensen T. S., “Neuropathic Pain: From Mechanisms to Treatment,” Physiological Reviews 101, no. 1 (2021): 259–301. - PubMed
-
- Disease G. B. D., Injury I., and Prevalence C., “Global, Regional, and National Incidence, Prevalence, and Years Lived With Disability for 328 Diseases and Injuries for 195 Countries, 1990‐2016: A Systematic Analysis for the Global Burden of Disease Study 2016,” Lancet 390, no. 10100 (2017): 1211–1259. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82171207/National Natural Science Foundation of China
- BK20231246/Natural Science Foundation of Jiangsu Province
- 2021-008/Youth Science and Technology Talents Support Project of Jiangsu Association for Science and Technology
- 2022-3-6-146/Youth Excellent Talent Training Project of "333" High-Level Personnel Training Program in Jiangsu Province
LinkOut - more resources
Full Text Sources
Miscellaneous

