Donanemab immunogenicity in participants with early symptomatic Alzheimer's disease
- PMID: 40904419
- PMCID: PMC12402809
- DOI: 10.1002/trc2.70149
Donanemab immunogenicity in participants with early symptomatic Alzheimer's disease
Abstract
Introduction: Donanemab is an immunoglobulin G1 antibody that targets an N-terminal truncated form of amyloid beta present in mature plaques. Treatment-emergent (TE) anti-drug antibodies (ADAs) were quantified in donanemab-treated participants from two pivotal clinical trials, and effects of TE ADAs on donanemab pharmacokinetics, efficacy, and safety were assessed.
Methods: Data were pooled from the phase 2 TRAILBLAZER-ALZ (NCT03367403) and phase 3 TRAILBLAZER-ALZ 2 trials (NCT04437511). Eligible participants were randomized 1:1 to donanemab (700 mg for the first three doses, 1400 mg thereafter) or placebo intravenously every 4 weeks up to 72 weeks. TE ADA-evaluable participants had a non-missing baseline ADA result and ≥ 1 non-missing post-baseline ADA result. TE ADA incidence and effect of titer on pharmacokinetics, amyloid plaque reduction, clinical efficacy (measured by change from baseline of integrated Alzheimer's Disease Rating Scale [iADRS] score and Clinical Dementia Rating Scale Sum of Boxes [CDR-SB]), and safety were assessed.
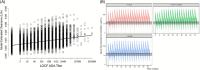
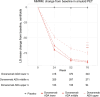
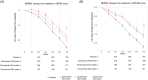
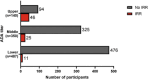
Results: Of 922 TE ADA-evaluable donanemab-treated participants, 56 (6.1%) had ADAs detected at baseline, and 812 (88.1%) were TE ADA positive. Donanemab clearance increased linearly with logarithm of ADA titer; however, titer did not affect maximum donanemab concentration. Amyloid plaque level was significantly reduced with donanemab versus placebo, irrespective of titer (P < 0.001 for all). No association was found between ADA presence or titer and donanemab efficacy by iADRS or CDR-SB. Eighty-four of 984 (8.5%) donanemab-treated participants and 4 of 999 (0.4%) placebo-treated participants reported infusion-related reactions (IRRs). All donanemab-treated participants reporting immediate IRRs developed ADAs at some point during the study; however, 90.5% of TE ADA-positive participants did not experience IRRs.
Discussion: Most participants were TE ADA positive. TE ADAs increased donanemab clearance but did not have clinically meaningful impact on plaque reduction or efficacy. While all participants reporting IRRs developed ADAs at some point during the study, the majority of participants with ADAs did not experience IRRs.
Highlights: In pivotal trials, most donanemab-treated participants were treatment-emergent anti-drug antibody (TE ADA) positive.TE ADAs increased donanemab clearance but did not impact plaque reduction/efficacy.All participants reporting infusion-related reactions (IRRs) developed ADAs at some point during the study.However, the majority of participants with ADAs did not experience IRRs.
Keywords: Alzheimer's disease; amyloid plaques; anti‐drug antibodies; donanemab; immunogenicity.
© 2025 Eli Lilly and Company. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
G.R.M., P.A., I.G., J.B., L.C., J.A.Z., C.D.E., E.S.M.N., H.W., R.K., D.A.B., and J.R.S. are employees and shareholders of Eli Lilly & Company, which funded this research. G.A. was an employee of Eli Lilly & Company at the time this research was completed. Author disclosures are available in the supporting information.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
