Gut microbiota and ankylosing spondylitis: current insights and future challenges
- PMID: 40904690
- PMCID: PMC12404692
- DOI: 10.15698/mic2025.08.857
Gut microbiota and ankylosing spondylitis: current insights and future challenges
Abstract
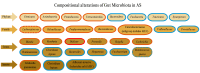
Ankylosing spondylitis (AS) is a chronic inflammatory disease with complex pathogenesis influenced by genetic, immunological and environmental factors. Recent evidence suggests that gut microbiota significantly contributes to AS etiopathogenesis. Dysbiosis and altered immune responses in the gut potentially trigger or exacerbate the disease through intestinal barrier disruption, alteration of the IL-23/17 axis and metabolite production. This review explores the growing role of gut microbiota in AS and its potential to reshape targeted treatment strategies and facilitate development of adjunct therapies to address disease onset and progression. AS is a multifactorial disease in which gut dysbiosis plays a significant role influencing immune regulation notably through the IL-23/17 pathway. Alterations in gut microbiota composition and its metabolites contribute to systemic inflammation, reinforcing a self-perpetuating feedback loop between gut and spinal inflammation that drives disease progression. Emerging evidence has linked microbial mechanisms to HLA-B27 misfolding promoting endoplasmic reticulum stress and triggering molecular mimicry through gut microbial-associated molecular patterns further contributing to AS pathogenesis. Given the crucial role of gut microbiota in AS, targeting microbiota imbalances presents a promising avenue for novel therapeutic strategies. Although it remains unclear whether gut inflammation and microbial changes precedes AS onset, current evidence suggests an ongoing cycle of autoimmune inflammation involving both the gut and joints. Further research, particularly longitudinal studies, are needed to better understand the gut-joint axis and its potential therapeutic implications in AS management.
Keywords: HLA-B27; IL-23/17 axis; autoimmune disease; dysbiosis; gut-joint axis; microorganisms; spondyloarthritis.
Copyright: © 2025 Lobiuc et al.
Conflict of interest statement
The authors declare that there is no conflict of interest concerning the publication of this manuscript.
Figures



References
-
- Bohn R, Cooney M, Deodhar A, Curtis JR, Golembesky A. Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol. 2018;36(2):263–274. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
