Three Biologic-Naïve Patients with Allergic Bronchopulmonary Aspergillosis Showing Significant Clinical Improvement with Tezepelumab
- PMID: 40904741
- PMCID: PMC12402420
- DOI: 10.2147/JAA.S541770
Three Biologic-Naïve Patients with Allergic Bronchopulmonary Aspergillosis Showing Significant Clinical Improvement with Tezepelumab
Abstract
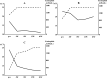
Allergic bronchopulmonary aspergillosis is characterized by hypersensitivity to Aspergillus spp. and often causes intractable asthma. Studies have been conducted on biologics administered to patients with allergic bronchopulmonary aspergillosis; however, treatment may not always be successful. This may be due to the limitations of the current biologics that selectively target a single cytokine. Tezepelumab, a human monoclonal antibody that blocks the activity of thymic stromal lymphopoietin, broadly suppresses type 2 inflammation by regulating the upstream cascade of airway inflammation. Therefore, it is expected to have favorable effects in patients with allergic bronchopulmonary aspergillosis. We report three cases of allergic bronchopulmonary aspergillosis with uncontrolled symptoms despite the maximal use of conventional anti-asthmatic drugs such as inhalative agents, anti-leukotriene receptor antagonists, and antifungal drugs. None of the patients had previously received biologics. The addition of tezepelumab produced a marked clinical response in all three patients, which included fewer exacerbations and a reduced dosage of oral systemic corticosteroids and/or reduced as-needed short-acting beta-2 agonists. The patients' pulmonary symptoms were better controlled, peripheral blood eosinophil counts and immunoglobulin E levels decreased, and quality of life scores and respiratory function parameters improved. Mucous plugs accompanied by atelectasis and infiltrative shadows observed on chest computed tomography also improved. Tezepelumab may be a promising treatment option for allergic bronchopulmonary aspergillosis in patients with severe asthma, offering effective symptom control and enabling reduction in systemic corticosteroid use.
Keywords: mucous plug; peripheral blood eosinophilia; severe asthma; type 2 inflammatory biomarker.
© 2025 Yamaguchi et al.
Conflict of interest statement
M.Y. receives lecture fees from AstraZeneca, GlaxoSmithKline, Sanofi, and Kyorin. H.N. receives lecture fees from Boehringer Ingelheim. Y.N. receives lecture fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Teijin Pharma. The authors declare no other conflicts of interest in this work.
Figures

Similar articles
-
[Guidelines for the prevention and management of bronchial asthma (2024 edition)].Zhonghua Jie He He Hu Xi Za Zhi. 2025 Mar 12;48(3):208-248. doi: 10.3760/cma.j.cn112147-20241013-00601. Zhonghua Jie He He Hu Xi Za Zhi. 2025. PMID: 40050074 Chinese.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Oral and intranasal aspirin desensitisation for non-steroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease.Cochrane Database Syst Rev. 2025 Jan 7;1(1):CD013476. doi: 10.1002/14651858.CD013476.pub2. Cochrane Database Syst Rev. 2025. PMID: 39775459
-
Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis.Cochrane Database Syst Rev. 2018 Mar 18;3(3):CD010288. doi: 10.1002/14651858.CD010288.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 Sep 22;9:CD010288. doi: 10.1002/14651858.CD010288.pub5. PMID: 29551015 Free PMC article. Updated.
-
Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis.Cochrane Database Syst Rev. 2015 Nov 4;(11):CD010288. doi: 10.1002/14651858.CD010288.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Mar 18;3:CD010288. doi: 10.1002/14651858.CD010288.pub4. PMID: 26545165 Updated.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

