Synthetic thermoresponsive scaffolds for the expansion and differentiation of human pluripotent stem cells into cardiomyocytes
- PMID: 40904843
- PMCID: PMC12402885
- DOI: 10.1039/d5ra04674b
Synthetic thermoresponsive scaffolds for the expansion and differentiation of human pluripotent stem cells into cardiomyocytes
Abstract
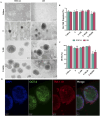
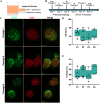
The advancement of regenerative medicine requires robust, reproducible, and scalable platforms for the expansion and differentiation of human pluripotent stem cells (hPSCs) into specialized cells, such as cardiomyocytes. While current natural matrices like Matrigel™ suffer from batch-to-batch variability and limited tunability, synthetic scaffolds with controllable biochemical and mechanical properties could provide superior platforms for maintaining stem cell pluripotency and directing efficient cardiac differentiation. Here, we report the development and evaluation of a customizable thermoresponsive terpolymer composed of N-isopropylacrylamide (NiPAAm), vinylphenylboronic acid (VPBA), and polyethylene glycol monomethyl ether monomethacrylate (PEGMMA) synthesized via free-radical polymerization as a synthetic matrix for human hPSC culture. This terpolymer exhibited optimal properties, including tunable stiffness, transparency, and thermoresponsiveness, facilitating non-invasive cell harvesting and downstream applications, characterized by flow cytometry, immunofluorescence, and gene expression analysis. In both two-dimensional (2D) and three-dimensional (3D) culture conditions, the terpolymer effectively supported the maintenance of pluripotency and promoted robust proliferation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells (hESCs). Furthermore, incorporation of bioactive molecules into the synthetic matrix such as RGD peptides, vitronectin, and fibronectin significantly enhanced cell expansion, aggregation, and cardiac differentiation efficiency. Differentiated cardiomyocytes exhibited statistically significant increases in the expression of cardiac-specific markers (cTnT and cTnI) reaching ∼65% and ∼25% respectively, compared to cells cultured on traditional matrices like Matrigel™, Cultrex™ and synthetic VitroGel™. These findings demonstrated the potential of this synthetic terpolymer scaffold as a reliable and scalable alternative for hPSC culture and differentiation, with significant implications for regenerative medicine, drug screening, and cardiac disease modeling.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
This work is the subject of intellectual property filings by the University of Puerto Rico, including a pending patent titled “Synthetic Matrixes for Cell Culture and Manufacture” (PCT/US2021/061256) and an invention disclosure titled “Synthetic Polymer Blends with Broad Mechanical Properties for Cell Culture” (25-007-DISC-UPR).
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

