Advances in applied supramolecular technologies 2021-2025
- PMID: 40905105
- PMCID: PMC12409610
- DOI: 10.1039/d4cs01037j
Advances in applied supramolecular technologies 2021-2025
Abstract
Supramolecular chemistry is a rapidly evolving field that has focused on building a foundation of fundamental understanding in controlling molecular self-assembly, through the use of non-covalent interactions. A common criticism of the field is that whilst the systems produced are very elegant, they do not have real-world use. Therefore, focus is now moving to applying the fundamental understanding of supramolecular chemistry to the production of commercially viable products. Building on our previous review in this area, which described the translational potential of innovations within the field of supramolecular chemistry up to the year 2020, we now review the progress of this field over the years 2021-2025 with the aim to inspire researchers to apply supramolecular chemistry to solve real world problems, moving innovation out of the laboratory and into the commercial marketplace.
Conflict of interest statement
PAG is a member of Chemical Society Reviews Advisory Board and is a former Chair of the Editorial Board.
Figures




















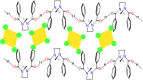

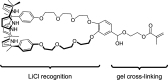
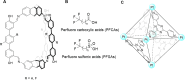
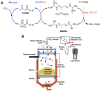









References
-
- Lehn J. M. Acc. Chem. Res. 1978;11:49–57. doi: 10.1021/ar50122a001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

