Lithocholic acid ameliorates ulcerative colitis via the PXR/TLR4/NF-κB/NLRP3 signaling pathway and gut microbiota modulation
- PMID: 40905977
- PMCID: PMC12411392
- DOI: 10.1007/s00018-025-05834-2
Lithocholic acid ameliorates ulcerative colitis via the PXR/TLR4/NF-κB/NLRP3 signaling pathway and gut microbiota modulation
Erratum in
-
Correction: Lithocholic acid ameliorates ulcerative colitis via the PXR/TLR4/NF-κB/NLRP3 signaling pathway and gut microbiota modulation.Cell Mol Life Sci. 2025 Nov 12;82(1):392. doi: 10.1007/s00018-025-05919-y. Cell Mol Life Sci. 2025. PMID: 41222670 Free PMC article. No abstract available.
Abstract
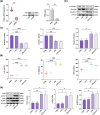
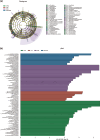
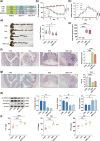
Ulcerative colitis (UC) is a chronic inflammatory condition of the colon, closely linked to dysbiosis of gut microbiota and imbalances in bile acids. Lithocholic acid (LCA), a secondary bile acid, plays a crucial role in maintaining gut health; however, its specific therapeutic potential in UC remains to be fully elucidated. This study investigates the efficacy of LCA in alleviating UC and explores the underlying mechanisms, particularly focusing on the PXR/TLR4/NF-κB/NLRP3 signaling pathway and gut microbiota modulation. Using a dextran sulfate sodium (DSS)-induced colitis model, our findings demonstrate that LCA administration significantly alleviates colitis symptoms, evidenced by reduced disease activity index (DAI), increased colon length, improved intestinal barrier function, and decreased colonic inflammation. Mechanistically, LCA activates the pregnane X receptor (PXR), which inhibits TLR4-mediated NF-κB/NLRP3 inflammasome activation, leading to reduced colonic inflammation and lower levels of pro-inflammatory cytokines. Furthermore, LCA remodels gut microbiota by promoting beneficial bacterial growth, such as Akkermansiaceae, Lactobacillaceae and Muribaculaceae, while suppressing pathogenic and opportunistic pathogens, including Enterobacteriaceae and Bacteroidaceae. The gut microbiota-dependent effects of LCA were corroborated through antibiotic treatment and fecal microbiota transplantation (FMT) experiments. Notably, the absence of intestinal flora affected PXR expression and activity, modifying the aforementioned effects. Overall, our findings reveal that LCA ameliorates experimental colitis by regulating the PXR/TLR4/NF-κB/NLRP3 signaling cascade and modulating gut microbiota composition. This study underscores LCA's potential as a targeted therapeutic strategy and a promising microbiota-focused approach for managing UC, offering new insights into the role of bile acids in intestinal health and disease management.
Keywords: Gut microbiota; Lithocholic acid; NF-κB; NLRP3; Pregnane X receptor; TLR4; Ulcerative colitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no conflicts of interest. Ethical statement: All animal care and experimental procedures were approved by the Animal Research Committee of Southeast University School of Medicine (Nanjing, China) (No. 20240223001).
Figures






References
-
- Le Berre C, Honap S, Peyrin-Biroulet L (2023) Ulcerative colitis. Lancet Lond Engl 402(10401):571–584. 10.1016/S0140-6736(23)00966-2 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

