Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients
- PMID: 40906100
- PMCID: PMC12383909
- DOI: 10.3390/biology14080944
Distribution Patterns and Assembly Mechanisms of Rhizosphere Soil Microbial Communities in Schisandra sphenanthera Across Altitudinal Gradients
Abstract
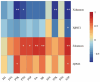
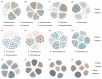
To investigate the characteristics of rhizosphere soil microbial communities associated with Schisandra sphenanthera across different altitudinal gradients and to reveal the driving factors of microbial community dynamics, this study collected rhizosphere soil samples at four elevations: 900 m (HB1), 1100 m (HB2), 1300 m (HB3), and 1500 m (HB4). High-throughput sequencing and molecular ecological network analysis were employed to analyze the microbial community composition and species interactions. A null model was applied to elucidate community assembly mechanisms. The results demonstrated that bacterial communities were dominated by Proteobacteria, Acidobacteriota, Actinobacteriota, and Chloroflexi. The relative abundance of Proteobacteria increased with elevation, while that of Acidobacteriota and Actinobacteriota declined. Fungal communities were primarily composed of Ascomycota and Basidiomycota, with both showing elevated relative abundances at higher altitudes. Diversity indices revealed that HB2 exhibited the highest bacterial Chao, Ace, and Shannon indices but the lowest Simpson index. For fungi, HB3 displayed the highest Chao and Ace indices, whereas HB4 showed the highest Shannon index and the lowest Simpson index. Ecological network analysis indicated stronger bacterial competition at lower elevations and enhanced cooperation at higher elevations, contrasting with fungal communities that exhibited increased competition at higher altitudes. Altitude and soil nutrients were negatively correlated with soil carbon content, while plant nutrients and fungal diversity positively correlated with soil carbon. Null model analysis suggested that deterministic processes dominated bacterial community assembly, whereas stochastic processes governed fungal assembly. These findings highlight significant altitudinal shifts in the microbial community structure and assembly mechanisms in S. sphenanthera rhizosphere soils, driven by the synergistic effects of soil nutrients, plant growth, and fungal diversity. This study provides critical insights into microbial ecology and carbon cycling in alpine ecosystems, offering a scientific basis for ecosystem management and conservation.
Keywords: Schisandra sphenanthera; altitude; co-occurrence network; community assembly; soil microorganisms.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analysis, and interpretation of data; or in writing the manuscript.
Figures






Similar articles
-
Structure and function of rhizosphere soil microbial communities associated with root rot of Knoxia roxburghii.Front Microbiol. 2024 Jul 18;15:1424633. doi: 10.3389/fmicb.2024.1424633. eCollection 2024. Front Microbiol. 2024. PMID: 39091303 Free PMC article.
-
Environmental Factors Drive the Changes of Bacterial Structure and Functional Diversity in Rhizosphere Soil of Hippophae rhamnoides subsp. sinensis Rousi in Arid Regions of Northwest China.Microorganisms. 2025 Aug 8;13(8):1860. doi: 10.3390/microorganisms13081860. Microorganisms. 2025. PMID: 40871364 Free PMC article.
-
Host-Determined Diversity and Environment-Shaped Community Assembly of Phyllosphere Microbiomes in Alpine Steppes Ecosystems.Microorganisms. 2025 Jun 19;13(6):1432. doi: 10.3390/microorganisms13061432. Microorganisms. 2025. PMID: 40572320 Free PMC article.
-
[Effect of Biochar-based Fertilizer Application on Soil Enzyme Activity, Fungal Community, and Crop Yield in Winter Wheat-Summer Maize Rotation Farmland].Huan Jing Ke Xue. 2025 Jun 8;46(6):3965-3974. doi: 10.13227/j.hjkx.202405297. Huan Jing Ke Xue. 2025. PMID: 40582831 Chinese.
-
Elevation-dependent shifts in soil phosphorus forms and phosphorus-solubilizing microbial diversity suggest enhanced bioavailable phosphorus cycling with rising temperatures.Microbiol Spectr. 2025 Aug 5;13(8):e0130024. doi: 10.1128/spectrum.01300-24. Epub 2025 Jul 9. Microbiol Spectr. 2025. PMID: 40631746 Free PMC article.
References
-
- Szopa A., Barnaś M., Ekiert H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2019;18:109–128. doi: 10.1007/s11101-018-9582-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

