Pathophysiology of Femoral Fractures in Hypophosphatasia
- PMID: 40906226
- PMCID: PMC12411579
- DOI: 10.1007/s11914-025-00929-y
Pathophysiology of Femoral Fractures in Hypophosphatasia
Abstract
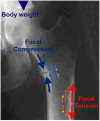
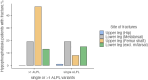
Purpose of review: In this review, we will examine the pathophysiology, anatomy, biochemistry, and genotype-phenotype correlation of femoral fractures in adult hypophosphatasia.
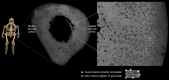
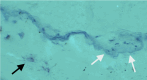
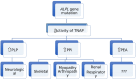
Recent findings: Hypophosphatasia (HPP) is a rare genetic disease characterized by low activity of tissue-nonspecific alkaline phosphatase (TNAP). The disease presents a broad spectrum of clinical manifestations primarily determined by the degree of residual TNAP activity. Adults with HPP of moderate clinical severity may present with spontaneous femoral fractures that are like the atypical femoral fractures (AFF) of long-term bisphosphonates users. In this review, we will focus on the paradox that while HPP can cause biopsy-proven osteomalacia (pathologically impaired bone mineralisation), the spontaneous femoral fractures that characterise adult HPP do not exhibit typical osteomalacia features. Instead, they resemble the femoral fractures that occur in other diseases such as osteopetrosis where bone becomes excessively dense, brittle and highly mineralised due to osteoclast dysfunction. This review examines the key aspects of the pathophysiology of femoral fractures in adults with HPP, offering new insights into the role of anatomical, molecular and biochemical bone abnormalities that characterise the disease. Further investigations of HPP patients with femoral fracture are needed to examine the nanoscale crystal structure of the bone and to study abnormalities in fracture healing and bone resorption.
Keywords: Bone mineralization; Femoral fractures; Hypophosphatasia; Osteomalacia; Osteopetrosis; TNAP.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate: The patients described in this review were identified using the Cambridge Outpatient Bone Registry (COBRA), as part of the East Anglian Rare Bone Network (ErBON) and gave informed consent for publication. The study “Characterisation of the mineral content of bone in hypophosphatasia” was approved by the Research Ethics Committee and HRA 22/HRA/0246. Competing Interests: The authors have no competing interests to declare that are relevant to the content of this article.
Figures

References
-
- Weiss MJ, Ray K, Henthorn PS, Lamb B, Kadesch T, Harris H. ‘Structure of the human liver/bone/kidney alkaline phosphatase gene’. J Biol Chem, 263, 24, pp. 12002–10 PMID– 3165380, 1988. - PubMed
-
- Whyte MP. Hypophosphatasia: an overview for 2017. Bone. 2017;102:15–25. 10.1016/j.bone.2017.02.011. - PubMed
-
- Fallon MD, Teitelbaum SL, Weinstein RS, Goldfischer S, Brown DM, Whyte MP. Hypophosphatasia: clinicopathologic comparison of the infantile, childhood, and adult forms. Med (United States). 1984;63(1). 10.1097/00005792-198401000-00002. - PubMed
-
- Berkseth KE, Tebben PJ, Drake MT, Hefferan TE, Jewison DE, Wermers RA. ‘Clinical spectrum of hypophosphatasia diagnosed in adults’, Bone, vol. 54, no. 1, pp. 21–27, 2013, 10.1016/j.bone.2013.01.024 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

