Deciphering the Gene Expression and Alternative Splicing Basis of Muscle Development Through Interpretable Machine Learning Models
- PMID: 40906371
- PMCID: PMC12383657
- DOI: 10.3390/biology14081059
Deciphering the Gene Expression and Alternative Splicing Basis of Muscle Development Through Interpretable Machine Learning Models
Abstract
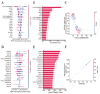
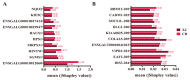
In chickens, meat yield is a crucial trait in breeding programs. Identifying key molecular markers associated with increased muscle yield is essential for breeding strategies. This study applied transcriptome sequencing and machine learning methods to examine gene expression and alternative splicing (AS) events in muscle tissues of commercial broilers and local chickens. On the basis of differentially expressed genes (DEGs) and differentially spliced transcripts (DSTs) significantly related to breast muscle weight percentage (BrP), high-accuracy prediction models were developed by evaluating 10 machine learning models (e.g., eXtreme Gradient Boosting (XGBoost), Generalized Linear Model Network (Glmnet)). Feature importance was assessed using the Shapley Additive exPlanations (SHAP) method. The results revealed that 50 DEGs and 95 DSTs contributed significantly to BrP prediction. The XGBoost model achieved over 90% accuracy when using DEGs, and the Glmnet model reached 95% accuracy when using DSTs. Through Shapley evaluation, genes and AS events (e.g., ENSGALG00010012060, HINTW, and VIPR2-201) were identified as having the highest contributions to BrP prediction. Additionally, the breed effect was effectively mitigated. This study introduces new candidate genes and AS targets for the molecular breeding of poultry breast muscle traits, offering a paradigm shift from traditional gene mining approaches to artificial intelligence-driven predictive methods.
Keywords: alternative splicing; breast muscle; chicken; machine learning; shapley additive exPlanations.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- FAOSTAT Statistical Database; FA. [(accessed on 30 May 2025)]. Available online: https://www.fao.org/faostat/en/#search/2019.
-
- Mottet A., Tempio G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017;73:245–256. doi: 10.1017/S0043933917000071. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

