Enhanced electrophysiological recordings in acute brain slices, spheroids, and organoids using 3D high-density multielectrode arrays
- PMID: 40906696
- PMCID: PMC12410755
- DOI: 10.1371/journal.pone.0328903
Enhanced electrophysiological recordings in acute brain slices, spheroids, and organoids using 3D high-density multielectrode arrays
Abstract
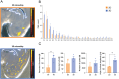
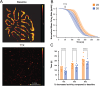
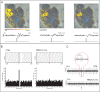
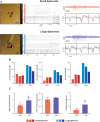
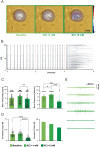
Recent advances in three-dimensional (3D) biological brain models in vitro and ex vivo are creating new opportunities to understand the complexity of neural networks but pose the technological challenge of obtaining high-throughput recordings of electrical activity from multiple sites in 3D at high spatiotemporal resolution. This cannot be achieved using planar multi-electrode arrays (MEAs), which contact just one side of the neural structure. Moreover, the specimen adhesion to planar MEAs limits fluid perfusion along with tissue viability and drug application. Here, the efficiency of the tissue-sensor interface provided by advanced 3D high-density (HD)-MEA technology was evaluated in acute brain slices, spheroids, and organoids obtained from different brain regions. The 3D HD-MEA microneedles reached the inner layers of samples without damaging network integrity and the microchannel network between microneedles improved tissue vitality and chemical compound diffusion. In acute cortico-hippocampal and cerebellar slices, signal recording and stimulation efficiency proved higher with the 3D HD-MEA than with a planar MEA improving the characterization of network activity and functional connectivity. The 3D HD-MEA also resolved the challenge of recording from brain spheroids as well as cortical and spinal organoids. Our results show that 3D HD-MEA technology represents a valuable tool to address the complex spatiotemporal organization of activity in brain microcircuits, making it possible to investigate 3D biological models.
Copyright: © 2025 Mapelli et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: MT, GS, CRB, are paid employees of 3Brain AG. MG, KI, and AM are shareholders of 3Brain AG. The 2D HD-MEA chip and 3D HD-MEA chip are products commercialized by 3Brain AG. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources

