Impact of Human Epidermal Growth Factor Receptor 2 in Patients With Metastatic Colorectal Cancer Treated With Chemotherapy Plus Bevacizumab or Anti-EGFRs: Exploratory Analysis of Eight Randomized Trials
- PMID: 40906970
- PMCID: PMC12509457
- DOI: 10.1200/JCO-25-01003
Impact of Human Epidermal Growth Factor Receptor 2 in Patients With Metastatic Colorectal Cancer Treated With Chemotherapy Plus Bevacizumab or Anti-EGFRs: Exploratory Analysis of Eight Randomized Trials
Abstract
Purpose: Human epidermal growth factor receptor 2 (HER2) amplification/overexpression (HER2-pos) is detected in 5% of RAS/BRAF wild-type metastatic colorectal cancers (mCRCs). Its prognostic/predictive role in terms of benefit from anti-EGFR/bevacizumab (bev) is debated. Similarly, the role of activating HER2 mutations (mut) is unclear.
Methods: We collected individual data of 1,604 patients with proficient mismatch repair (pMMR)/microsatellite stable (MSS) RAS/BRAF wild-type untreated mCRC with HER2 amplification/expression status available enrolled in eight randomized clinical trials (RCT; TRIBE2, TRIPLETE, VALENTINO, ATEZOTRIBE, PANDA, PANAMA, PARADIGM, and CALGB/SWOG80405). Objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were assessed with respect to HER2 amplification/expression and HER2 mutational status and according to biologics (anti-EGFR/bev).
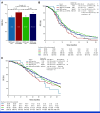
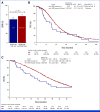
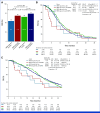
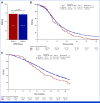
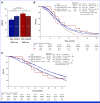
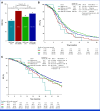
Results: Patients with HER2-pos were 81 (5%). HER2-pos patients experienced shorter PFS (median PFS [mPFS]: 9.8 v 12.2 months, hazard ratio [HR], 1.31, P = .02) and OS (median OS [mOS]: 28.0 v 34.9 months, HR, 1.37, P = .01), also after adjustment for covariates (PadjPFS = .02, PadjOS = .048). ORR was similar between HER2-pos and HER2-negative (HER2-neg) tumors (75% v 72%, odds ratio [OR], 1.21, P = .47). We found no interaction between HER2 amplification/expression status and biologics' effect in terms of PFS (Pint = .76), OS (Pint = .76), and ORR (Pint = .64). In left-sided HER2-pos tumors, outcomes were similar with chemotherapy plus bev/anti-EGFRs in terms of PFS (9.8 v 9.3 months, HR, 0.73, P = .29), OS (29.8 v 28.0 months, HR, 1.29, P = .40), and ORR (59% v 79%, OR, 0.39, P = .10). HER2-mutant tumors (2% of patients with HER2-neg tumors) showed shorter OS than HER2 wild-type ones (mOS: 23.7 v 34.4 months, HR, 1.56, P = .04) with no differential effect of biologics (PintORR = .81; PintPFS = .95; PintOS = .92).
Conclusion: To our knowledge, this is the largest analysis of HER2 status in patients with untreated mCRC enrolled in RCT. Waiting for targeted approaches, HER2-pos and mut do not predict benefit from bev/anti-EGFRs and should be regarded as negative prognostic factors in pMMR/MSS RAS/BRAF wild-type mCRC.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Ahcene Djaballah S, Daniel F, Milani A, et al. HER2 in colorectal cancer: The long and winding road from negative predictive factor to positive actionable target. Am Soc Clin Oncol Educ Book. 2022;42:219–232. - PubMed
-
- Venturini J, Massaro G, Lavacchi D, et al. The emerging HER2 landscape in colorectal cancer: The key to unveil the future treatment algorithm? Crit Rev Oncol Hematol. 2024;204:104515. - PubMed
-
- Valtorta E, Martino C, Sartore-Bianchi A, et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod Pathol. 2015;28:1481–1491. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

