Safety and Efficacy of Perispinal Etanercept for Chronic Stroke: A Randomized Clinical Trial
- PMID: 40906977
- PMCID: PMC12418806
- DOI: 10.1212/WNL.0000000000213981
Safety and Efficacy of Perispinal Etanercept for Chronic Stroke: A Randomized Clinical Trial
Abstract
Background and objectives: Stroke is a leading cause of long-term disability. Etanercept, a competitive tumor necrosis factor-α inhibitor, has been proposed as a potential treatment for post-stroke impairments when given through a perispinal subcutaneous injection. We aimed to evaluate the safety and efficacy of perispinal etanercept in patients with chronic stroke.
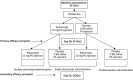
Methods: The Perispinal Etanercept for Stroke Outcomes Study was a randomized, double-blind, placebo-controlled, parallel group trial conducted in 3 ambulatory research clinics in Australia and New Zealand. Eligible patients were aged between 18 and 70 years, had a stroke between 1 and 15 years before enrollment, had a modified Rankin Scale score of 2-5, and demonstrated reduced quality of life as assessed by the 36-Item Short Form Survey (SF-36). Patients were randomized in a 1:1 ratio. The primary outcome was the difference in the proportion of participants at day 28 with an improvement of 5 points or more on the SF-36v2 compared with baseline after a single injection of etanercept 25 mg or equivalent placebo. The primary safety outcome was the difference in the proportion over 28 days of serious adverse events after a single injection of etanercept 25 mg or equivalent placebo. The primary outcome and safety were analyzed in the intention-to-treat population. Participants were followed up 56 days after the first injection of perispinal etanercept.
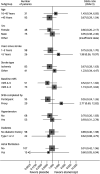
Results: Recruitment ceased early because of lack of continued funding. We screened 147 participants, of whom 126 were enrolled and randomized (63 etanercept: 63 placebo; 48 [38%] female, median (interquartile range) age 54.5 (45.0-60.0) years, median time since stroke 3 years). The primary outcome of improvement occurred in 33 of 63 participants (53%) in the etanercept arm and 36 of 63 participants (58%) in the placebo arm (adjusted odds ratio 0.82, 95% CI 0.40-1.67, standardized risk difference -5%, 95% CI -22% to 13%). The proportion of serious adverse events was similar in both treatment arms.
Discussion: Perispinal etanercept was safe, but we found no evidence of improvement in quality of life and other efficacy outcomes compared with placebo.
Trial registration information: The trial was registered at anzctr.org.au (ACTRN12620001011976) on October 7, 2020. Patients were enrolled between November 4, 2020, and September 1, 2023.
Classification of evidence: This study provides Class I evidence that in patients with imaging-confirmed ischemic or hemorrhagic stroke, perispinal etanercept (25 mg) injection does not improve the patients' quality of life 28 days after injection.
Conflict of interest statement
V.N. Thijs reports speaker fees for Amgen, Allergan, Astra Zeneca, Atricure, BMS, Bayer, Boehringer Ingelheim, and Medtronic, unrelated to this publication; is on the editorial board for
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials