Immunoprofiling at an Institutional Scale Reveals That High Numbers of Intratumoral CD8+ and PD-1+ Cells Predict Superior Patient Survival Across Major Cancer Types Independent of Major Risk Factors
- PMID: 40906987
- PMCID: PMC12419029
- DOI: 10.1200/PO-25-00240
Immunoprofiling at an Institutional Scale Reveals That High Numbers of Intratumoral CD8+ and PD-1+ Cells Predict Superior Patient Survival Across Major Cancer Types Independent of Major Risk Factors
Abstract
Purpose: Retrospective studies have found associations between the number of intratumoral immune cells and patient outcomes for specific cancers treated with targeted therapies. However, the clinical value of routinely quantifying intratumoral immune biomarkers using a digital pathology platform in the pan-cancer setting within an active clinical laboratory has not been established.
Methods: We developed ImmunoProfile, a daily clinical workflow that integrates automated multiplex immunofluorescence tissue staining, digital slide imaging, and machine learning-assisted scoring to quantify intratumoral CD8+, PD-1+, CD8+PD-1+, and FOXP3+ immune cells and PD-L1 expression in formalin-fixed, paraffin-embedded tissue samples in a standardized and reproducible manner. We prospectively applied ImmunoProfile to biopsies collected from 2,023 unselected patients with cancer over a 3-year period in the clinical laboratory and correlated the results with patient survival.
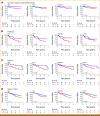
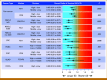
Results: In the pan-cancer cohort, patients with high numbers of intratumoral CD8+ or PD-1+ cells in had significantly lower risks of death compared with those with low numbers (CD8+: high v low hazard ratio [HR], 0.62 [95% CI, 0.48 to 0.81], Wald P = .002; PD-1+: high v low HR, 0.65 [95% CI, 0.51 to 0.83]; P = .0009) after adjusting for risk factors, including cancer type. In subset analyses, patients with high numbers of intratumoral CD8+, PD-1+, and/or CD8+PD-1+ cells showed lower risks of death from non-small cell lung, colorectal, breast, esophagogastric, head and neck, pancreatic, and ovarian cancers after considering clinical risk factors, including American Joint Committee on Cancer stage, and despite varying therapies (all P < .05).
Conclusion: Routinely quantifying intratumoral CD8+ and PD-1+ cells with a clinically validated digital pathology platform predicts patient survival across major cancer types, independent of clinical stage and despite diverse treatment regimens.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- Cree IA, Lokuhetty D, Tan PH: The World Health Organization classification of tumors and external quality assurance for immunohistochemistry and molecular pathology. Arch Pathol Lab Med 146:1303-1307, 2022 - PubMed
-
- Indave BI, Colling R, Campbell F, et al. : Evidence-levels in pathology for informing the WHO classification of tumours. Histopathology 81:420-425, 2022 - PubMed
-
- Andrici J, Gill AJ, Hornick JL: Next generation immunohistochemistry: Emerging substitutes to genetic testing? Semin Diagn Pathol 35:161-169, 2018 - PubMed
-
- Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144:646-674, 2011 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

