Patritumab deruxtecan in HR+HER2- advanced breast cancer: a phase 2 trial
- PMID: 40908353
- PMCID: PMC12532567
- DOI: 10.1038/s41591-025-03885-3
Patritumab deruxtecan in HR+HER2- advanced breast cancer: a phase 2 trial
Abstract
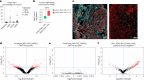
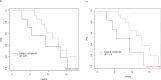
Antibody-drug conjugates have shown impressive clinical outcomes, particularly in metastatic breast cancer, but biomarkers to predict response and resistance remain unidentified. Here we report the results of ICARUS-BREAST01, a phase 2 study evaluating efficacy, safety and biomarkers of response and resistance to patritumab deruxtecan (HER3-DXd), in patients with HR+HER2- metastatic breast cancer, who previously progressed on CDK4/6 inhibitors and one line of chemotherapy. From May 2021 to June 2023, 99 patients were enrolled to receive HER3-DXd 5.6 mg kg-1 intravenously every 3 weeks. The study met its primary endpoint, showing an overall response rate of 53.5% (90% confidence interval [44.8-62.1%]). The most frequent adverse events were fatigue (83%), nausea (75%), diarrhea (53%) and alopecia (40%). Exploratory biomarker analysis of baseline tumor samples suggested preliminary associations between overall response rate and both HER3 spatial distribution and absence of estrogen receptor 1 (ESR1) mutations, as well as between progression-free survival and HER3 expression, pending further validation. Analysis of on-treatment tumor samples showed that treatment efficacy seems to be associated with antibody-drug conjugate intratumoral distribution and interferon response. Overall, HER3-DXd showed promising activity and manageable tolerability in patients with HR+HER2- metastatic breast cancer who progressed on CDK4/6 inhibitors. These findings highlight the need for larger trials to define HER3-DXd efficacy relative to other drugs, including antibody-drug conjugates (ClinicalTrials.gov Identifier: NCT04965766 ).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.P. has received consulting fees from AstraZeneca (institutional), Seagen (institutional), Gilead (institutional), Novartis (institutional), Lilly (institutional), MSD (institutional), Pierre Fabre (personal) and Daiichi Sankyo (institutional or personal); research funding (to the institution) from AstraZeneca, Daiichi Sankyo, Gilead, Seagen and MSD; and travel support from AstraZeneca, Pierre Fabre, MSD, Daiichi Sankyo and Pfizer. F.M. has received consulting fees from Novartis and Pegascy. M.L.T. has received consulting fees from AstraZeneca and Daiichi Sankyo. J.S.F. has received consulting fees, honoraria for lectures and presentations and support for attending meetings from and participation on data safety monitoring for AstraZeneca, GSK, Eisai, MSD, Lilly, Pfizer, Novartis, Daiichi Sankyo and Seagen. M.A.B. has received consulting fees from Daiichi Sankyo; honoraria for lectures and presentations from Exact Sciences; support for attending meetings from Novartis, Lilly and Seagen; and participation on data safety monitoring for MSD, AstraZeneca, Novartis, Lilly, Esai, Exact Sciences, Daiichi and Sankyo. T.B. has received grants or contracts from other entities, such as AstraZeneca (institution), Pfizer (institution), SeaGen (institution) and Novartis (institution); honoraria for lectures and presentations from SeaGen, Novartis, Pfizer and Lilly; support for attending meetings from Roche, AstraZeneca, Daiichi Sankyo, Pfizer and Novartis; and participation on data safety monitoring for AstraZeneca, Daiichi Sankyo, SeaGen, Novartis, Pfizer and Lilly. J.D. has received consulting fees from Daiichi Sankyo and AstraZeneca. A.S., F.S., L.L., D.W.S. and D.S. are employees of Daiichi Sankyo. S.M. is a data and safety monitoring member of clinical trials for IQVIA, Kedrion, Biophytis, Servier and Yuhan and a scientific committee study member of an observational study for Roche. F.A. has received research funding and speaker or advisor honoraria (compensated to the hospital) for Roche, AstraZeneca, Daiichi Sankyo, Pfizer, Novartis and Eli Lilly. The remaining authors declare no competing interests.
Figures







References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74, 12–49 (2024). - PubMed
-
- Sedeta, E. T., Jobre, B. & Avezbakiyev, B. Breast cancer: global patterns of incidence, mortality, and trends. J. Clin. Oncol.41, 10528–10528 (2023).
-
- Mosele, F., Montagnac, G., Pistilli, B. & André, F. Optimizing the potential of antibody–drug conjugates in oncology. Ann. Oncol.34, 964–967 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

