Changes in carriage and serotype diversity of Streptococcus pneumoniae and other respiratory pathobionts in the UK between pre-PCV13 (2006-10), early-PCV13 (2010-12) and late-PCV13 (2012-23) periods
- PMID: 40908474
- PMCID: PMC12412253
- DOI: 10.1186/s41479-025-00174-y
Changes in carriage and serotype diversity of Streptococcus pneumoniae and other respiratory pathobionts in the UK between pre-PCV13 (2006-10), early-PCV13 (2010-12) and late-PCV13 (2012-23) periods
Abstract
Background: The ongoing burden of mortality and morbidity associated with Streptococcus pneumoniae infections requires that monitoring of carriage epidemiology continues. Here, we present data from the annual, cross-sectional surveillance study in Southampton UK on serotype epidemiology and diversity, as well as carriage of other frequent colonisers of the respiratory tract in over 7000 children over a period of seventeen years (2006–2023).
Methods: Children were recruited from two sites: Site 1 - Southampton General Hospital, administered by University Hospital Southampton (UHS) NHS Foundation Trust and Site 2– a collection of community health care facilities within the Solent NHS Trust region. Recruitment was limited to children < 5-years-old. Pneumococcal serotyping was done using whole genome sequence data.
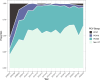
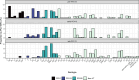
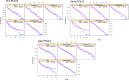
Results: A total of 7,686 swabs were collected from which 2,386 (31%) pneumococci were recovered. Carriage of pneumococci has remained consistent (median carriage prevalence 31.4%) even with the almost complete removal of vaccine-type (VT) serotypes. Examining three PCV13 periods separately (pre, early and late), carriage was not significantly different at 27.7%, 35.3% and 39.3% respectively. A decrease in carriage of Haemophilus influenzae, Staphylococcus aureus and Moraxella catarrhalis was seen pre-PCV13 (following PCV7 implementation) but has since stabilised. Continued, low-level persistence of VTs 3, 19A and 19F was noted. PCV13 did not impact the pneumococcal serotype rank abundance despite clear reductions in targeted serotypes and fluctuations in other non-VT serotypes such as 15A, 23B and 23B1. Non-PCV13 PCV20 serotypes 10A and 11A, in addition to paired prevalence of 15B and 15C (15B/C) were in the five most isolated serotypes in the late-PCV13 period (2012 to present). Non-PCV13 PCV20 serotypes now account for approximately 40% of all carriage. By contrast, the serotypes only included in PCV15 (22F and 33F) represented just 7% in the same period.
Conclusion: With consistent carriage prevalence in this UK paediatric population since PCV13 introduction, serotype epidemiology is now dominated by non-PCV13 serotypes that are in higher valency vaccines.
Supplementary Information: The online version contains supplementary material available at 10.1186/s41479-025-00174-y.
Keywords: Carriage epidemiology; PCV; Streptococcus pneumoniae.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The UK National Health Service (NHS) Research Ethics Service approved this study (06/Q1704/105 and 14/NS/1064). All methods and research practises outlined below were performed in accordance with relevant regulations and the Declaration of Helsinki. Written informed consent was provided by parents or legal guardians for each child. Consent for publication: Not applicable. Competing interests: DWC was a post-doctoral researcher on GSK funded projects in 2014/15, and currently receives grant support from Pfizer and the National Institute for Health via the NIHR Southampton Biomedical Research Centre. SNF receives support from the National Institute for Health Research funding via the NIHR Southampton Wellcome Trust Clinical Research Facility and the NIHR Southampton Biomedical Research Centre. SNF and SCC act as principal investigators for clinical trials and other studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers. No personal payments are received from them. SNF, JMJ and SCC have participated in advisory boards for vaccine manufacturers but receive no personal payments for this work. SNF, SCC and JMJ have received financial assistance from vaccine manufacturers to attend conferences. All grants and honoraria are paid into accounts within the respective NHS Trusts or Universities, or to independent charities. RAG, VTD and JJ received PhD studentships via the University of Southampton from Pfizer. RAG received post-doctoral support in 2012 on a GSK funded research project via the University of Southampton. KLO received PhD studentship support from GSK, again via the University of Southampton. JC, ML, KH, JS and BG are employees of Pfizer Inc and, as such, may hold stocks. All other authors have no conflicts of interest.
Figures




References
-
- Bertran M, D’Aeth JC, Abdullahi F, Eletu S, Andrews NJ, Ramsay ME, et al. Invasive pneumococcal disease 3 years after introduction of a reduced 1 + 1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: a prospective national observational surveillance study. Lancet Infect Dis. 2024;24(5):546–56. 10.1016/S1473-3099(23)00706-5. Epub 2024 Feb 1. Erratum in: Lancet Infect Dis.2024;24(6):e356. 10.1016/S1473-3099(24)00224-X. PMID: 38310905. - PubMed
-
- Sigurdsson S, Erlendsdóttir H, Quirk SJ, Kristjánsson J, Hauksson K, Andrésdóttir BDI, et al. Pneumococcal vaccination: direct and herd effect on carriage of vaccine types and antibiotic resistance in Icelandic children. Vaccine. 2017;35(39):5242–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

