A Randomized, Observer-Blinded, Active-Controlled, Phase IIIb Study to Compare IV/Oral Delafloxacin Fixed-Dose Monotherapy With Best Available Treatments in a Microbiologically Enriched Population With Surgical Site Infections: The DRESS Study
- PMID: 40908975
- PMCID: PMC12406695
- DOI: 10.1093/ofid/ofaf476
A Randomized, Observer-Blinded, Active-Controlled, Phase IIIb Study to Compare IV/Oral Delafloxacin Fixed-Dose Monotherapy With Best Available Treatments in a Microbiologically Enriched Population With Surgical Site Infections: The DRESS Study
Abstract
Background: Surgical site infections (SSIs) are the most common skin and skin structure infections and are mostly polymicrobial, requiring hospitalization and broad-spectrum antibiotics. This clinical trial evaluated the noninferiority of delafloxacin vs best available therapy (BAT) for the treatment of superficial or deep incisional SSI following a cardiothoracic/related leg or abdominal surgical procedure.
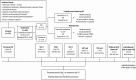
Methods: In this randomized, observer-blinded, active-controlled, parallel-group, multicenter, phase IIIb study, patients with SSI were randomized 1:1 to receive delafloxacin 300 mg intravenous (IV)/450 mg oral (OS) or BAT IV/OS (vancomycin or linezolid for cardiothoracic SSI, piperacillin/tazobactam or tigecycline for abdominal SSI). The primary end point was clinical success, defined as the clinical response (cure or improved) at test of cure (TOC), performed 7-14 days after end of treatment (EOT) visit. Secondary end points were clinical success at EOT, sustained clinical response at last follow-up (LFU), microbiological response, and safety.
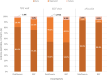
Results: Thi study enrolled 266 patients (delafloxacin = 134; BAT = 132) with comparable baseline characteristics between the 2 treatment arms. Delafloxacin clinical success was noninferior vs BAT at TOC visit (91.8% vs 90.2%, respectively). Similar efficacy was confirmed at LFU (91.8% delafloxacin; 87.9% BAT). Comparable microbiological response was obtained with delafloxacin (89.5%) and BAT (79.4%). Delafloxacin and BAT demonstrated comparable treatment adverse event rates (23.9% and 19.7%, respectively), mostly mild-to-moderate gastrointestinal reactions.
Conclusions: This study provided new data on delafloxacin in SSIs, covering the need for effective empiric treatment against the wide spectrum of pathogens involved in these infections.
Clinical trials registration: NCT04042077; 2018-001082-17 (EudraCT).
Keywords: acute bacterial skin and skin structure infections (ABSSSIs); clinical success; delafloxacin; safety; surgical site infections (SSIs).
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. G.M., A.N., B.M., S.S., G.M.M., and M.L. are employees of Menarini Ricerche SpA. All other authors report no potential conflicts.
Figures

References
-
- Esposito S, Noviello S, De Caro F, Boccia G. New insights into classification, epidemiology and microbiology of SSTIs, including diabetic foot infections. Infez Med 2018; 26:3–14. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

