Measurement of Amiodarone Levels in the Breast Milk of a Japanese Woman With Peripartum Cardiomyopathy
- PMID: 40909045
- PMCID: PMC12404898
- DOI: 10.7759/cureus.89301
Measurement of Amiodarone Levels in the Breast Milk of a Japanese Woman With Peripartum Cardiomyopathy
Abstract
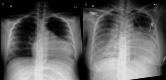
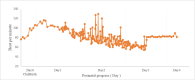
This study aimed to measure the concentrations of amiodarone (AM) and its active metabolite, mono-N-desethylamiodarone (DEA), in the breast milk of postpartum Japanese women treated with AM for ventricular tachycardia associated with peripartum cardiomyopathy and to conduct a follow-up study on the long-term growth and development of infants after resumption of breastfeeding. The patient was a 28-year-old Japanese woman with no underlying diseases who developed ventricular tachycardia and peripartum cardiomyopathy after giving birth. She was administered AM for three days via a combination of oral and intravenous administration. Breastfeeding began 35 days after the end of AM. Breast milk was collected 14 and 39 days after the end of AM, and its concentration in breast milk was measured using high-performance liquid chromatography. Furthermore, the growth and developmental test results of the twins were tracked for three years. Fourteen days after the end of AM administration, the AM concentration in breast milk was 111 ng/mL and the DEA concentration was 143 ng/mL. After 39 days, the AM concentration in breast milk was 11.8 ng/mL, and the DEA concentration in breast milk was 36.6 ng/mL. Studies have reported that pregnant women receiving AM are at risk of causing infant hypothyroidism and developmental delays due to the drug's high iodine content. Given the potential risks of invasive procedures and low milk intake, thyroid function testing was not conducted in the infant. Both twins were noted to be obese but had no clinical complications, and developmental evaluations showed no deficits. It was believed that resuming breastfeeding at that time was appropriate. Considering the risks associated with hypothyroidism, it is necessary to carefully determine the timing of breastfeeding initiation based on factors such as the mother's AM dosage, maternal blood concentration, and the infant's breast milk intake. However, it is considered acceptable for breastfeeding women who have received oral or intravenous AM for several days postpartum to breastfeed their infants.
Keywords: amiodarone; japanese; mono-n-desethyl amiodarone; peripartum cardiomyopathy; transferable into milk.
Copyright © 2025, Watanabe et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. The Akita Red Cross Hospital Ethics Review Committee issued approval 669, 768. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures




References
-
- Worldwide incidence of peripartum cardiomyopathy and overall maternal mortality. Isogai T, Kamiya CA. Int Heart J. 2019;60:503–511. - PubMed
-
- The increase in the rate of maternal deaths related to cardiovascular disease in Japan from 1991-1992 to 2010-2012. Tanaka H, Katsuragi S, Osato K, et al. J Cardiol. 2017;69:74–78. - PubMed
-
- Amiodarone in ventricular arrhythmias: still a valuable resource? Pannone L, D'Angelo G, Gulletta S, et al. Rev Cardiovasc Med. 2021;22:1383–1392. - PubMed
-
- JCS/JHRS 2020 guideline on pharmacotherapy of cardiac arrhythmias. Ono K, Iwasaki YK, Akao M, et al. Circ J. 2022;86:1790–1924. - PubMed
-
- Use of amiodarone during pregnancy. Plomp TA, Vulsma T, Vijlder JJ. Eur J Obstet Gynecol Reprod Biol. 1992;43:201–207. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
