Persistent progression independent of relapse activity in multiple sclerosis
- PMID: 40909095
- PMCID: PMC12405762
- DOI: 10.1093/braincomms/fcaf306
Persistent progression independent of relapse activity in multiple sclerosis
Abstract
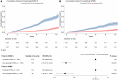
Patients with relapsing-remitting multiple sclerosis (RRMS) may experience disability progression independent of relapse activity (PIRA), which can be an early sign of secondary progressive MS (SPMS). We defined persistent PIRA as ongoing sustained disability over the entire available follow-up period. However, PIRA events can regress over time. Identifying factors that predict PIRA persistence is of great interest as they can refine the definition of RRMS to SPMS transition. Equally, factors associated with the non-persistence of PIRA have potential treatment implications for patients suffering from a PIRA event. We conducted a study to examine risk factors for PIRA persistence and risk differences in long-term disability progression between persistent and non-persistent PIRA. In this cohort study, we included only patients who had already experienced a PIRA event and investigated the persistence of disability progression following their first PIRA event. Therefore, PIRA occurrence time was set as the baseline. Data were collected from the MSBase registry between April 1995 and January 2024, with a median follow-up of 8.7 years. The primary outcome was time to 6-month confirmed non-persistence of PIRA. Secondary outcomes comprised time to 6-month confirmed Expanded Disability Status Scale (EDSS) 6 and time to SPMS. A stratified Cox regression model was used to identify risk factors associated with non-persistent PIRA. We then matched persistent PIRA patients with non-persistent PIRA patients in a 1:1 ratio using propensity scores, and compared their risk of reaching EDSS 6 using the Cox regression model. We re-matched patients with complete Kurtzke Functional Systems Scores to compare their risks of reaching SPMS. We included 4713 RRMS patients with PIRA, of whom around one-third experienced a post-PIRA disability improvement, over a relatively long period (median of 2.6 years to improvement). Use of high-efficacy disease-modifying therapies (DMT) at baseline [hazard ratio, 1.22; 95% confidence interval, (1.08-1.38); P = 0.0015], lower baseline EDSS [hazard ratio, 0.73 (0.69-0.78); P < 0.0001] and younger age [per 10 years; hazard ratio, 0.84 (0.80-0.89); P < 0.0001] were associated with non-persistent PIRA. Patients with non-persistent PIRA had a hazard ratio of 0.19 [95% confidence interval, (0.15-0.25); P < 0.0001] for reaching EDSS 6 and 0.18 [(0.11-0.29); P < 0.0001] for reaching SPMS compared to patients with persistent PIRA. PIRA events slowly regress in one-third of patients. Patients with persistent PIRA had a substantially higher risk of reaching EDSS 6 and SPMS than those with non-persistent PIRA. Younger age, lower baseline EDSS, and use of high-efficacy DMT during PIRA events were associated with PIRA regression.
Keywords: disability improvement; disability progression; progression independent of relapse activity (PIRA); relapsing-remitting multiple sclerosis (RRMS); secondary progressive MS (SPMS).
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
Chao Zhu, Zhen Zhou, Marc Girard, Oliver Gerlach, Elisabetta Cartechini, Emanuele D'Amico, and Serkan Ozakbas report no disclosures. Daniel Merlo has received honoraria from Novartis. Mastura Monif reported serving on the advisory board for Merck; receiving speaker honoraria from Merck and Biogen; and receiving funding from Merck, the Australian National Health Medical Research Council, Brain Foundation, Charles and Sylvia Viertel Foundation, Bethlehem Griffith Foundation, and Multiple Sclerosis (MS) Research Australia. Katherine Buzzard has received honoraria for presentations and/or educational support from Biogen, Sanofi Genzyme, Merck, Roche, Alexion and Teva. She serves on medical advisory boards for Merck and Biogen. Olga Skibina received honoraria and consulting fees from Bayer Schering, Novartis, Merck, Biogen and Genzyme. Raed Alroughani received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GlaxoSmithKline, Merck, Novartis, Roche and Sanofi-Genzyme. Pierre Grammond has served in advisory boards for Novartis, EMD Serono, Roche, Biogen idec, Sanofi Genzyme, and Pendopharm and has received grant support from Genzyme and Roche, has received research grants for his institution from Biogen idec, Sanofi Genzyme, EMD Serono. Jeannette Lechner-Scott received travel compensation from Novartis, Biogen, Roche and Merck. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Biogen, Merck, Roche, Teva, and Novartis. Tomas Kalincik served on scientific advisory boards for the MS International Federation and World Health Organization, Bristol Myers Squibb, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, the steering committee for the Brain Atrophy Initiative by Sanofi Genzyme, and has received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL and Merck, and research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. Nevin John is a primary investigator on commercial MS studies sponsored by Novartis, Roche, Biogen and Sanofi. He has received speaker’s honoraria from Merck. He has had conference travel and registration reimbursement from Novartis. Pamela McCombe received speakers fees and travel grants from Novartis, Biogen, T’évalua, and Sanofi. Richard Macdonell or his institution have received remuneration for his speaking engagements, advisory board memberships, research and travel from Biogen, Merck, Genzyme, Bayer, Roche, Teva, Novartis, CSL, BMS, MedDay and National Health and Medical Research Council (NHMRC). Izanne Roos has served on scientific advisory boards, received conference travel support and/or speaker honoraria from Roche, Novartis, Merck and Biogen. She is supported by MS Australia and the Trish Multiple Sclerosis Research Foundation. Vincent van Pesch received travel grants from Merck Healthcare KGaA (Darmstadt, Germany), Biogen, Sanofi, Bristol Meyer Squibb, Almirall and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Merck Healthcare KGaA (Darmstadt, Germany), Bristol Meyer Squibb, Janssen, Almirall, Novartis Pharma, and Alexion. Guy Laureys received travel and/or consultancy compensation from Sanofi-Genzyme, Roche, Teva, Merck, Novartis, Celgene, and Biogen. Julie Prevost accepted travel compensation from Novartis, Biogen, Genzyme, Teva, and speaking honoraria from Biogen, Novartis, Genzyme and Teva. Jens Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi, Stata DX. Dana Horakova was supported by the Charles University: Cooperatio Program in Neuroscience, by the project National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107)—Funded by the European Union—Next Generation EU, and by General University Hospital in Prague project MH CZ-DRO-VFN64165. She also received compensation for travel, speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck, Bayer, Sanofi Genzyme, Roche, and Teva, as well as support for research activities from Biogen Idec. Eva Kubala Havrdova received honoraria/research support from Biogen, Merck Serono, Novars, Roche, and Teva; has been member of advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novars, and Sanofi Genzyme; and has been supported by the Czech Ministry of Education—project Cooperatio LF1, research area Neuroscience, and the project National Institute for Neurological Research (Programme EXCELES, ID project No LX22NPO5107)—funded by the European Union-Next Generation EU. Tamara Castillo-Triviño received speaking/consulting fees and/or travel funding from Almirall, Biogen, Bristol Myers Squibb, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Cristina Ramo-Tello has received consulting fees, speaker honoraria, suport for attending meetings and/or travel, participation on advisory board and research grants for her institution from Biogen, Novartis, Sanofi, Bristol, Roche, Almirall, Janssen, Sandoz and Merck. Yolanda Blanco received speaker honoraira/consulting fees from Merck, Biogen, Roche, Brystol, Novartis, Sanofi and Sandoz. Jose E Meca-Lallana has received grants and consulting or speaking fees from Alexion, Almirall, Biogen, Bristol-Meyers-Squibb, Horizon, Janssen, Merck, Novartis, Roche, Sandoz and Sanofi. Alessandra Lugaresi has received personal compensation for consulting, serving on a scientific advisory board, speaking or other activities from Alexion, Biogen, Bristol Myers Squibb, Horizon, Janssen, Merck Serono, Novartis, and Sanofi/Genzyme, and Her institutions have received research grants from Novartis and Sanofi/Genzyme. Valentina Tomassini has received consultation and speaker fees, travel grants and research support from: Biogen, Sanofi Genzyme, Merck, Novartis, Roche, Alexion, Viatris, Janssen, Bristol Myers Squibb, Almirall. Maria Pia Amato received honoraria as consultant on scientific advisory boards by Biogen, Bayer-Schering, Merck, Teva and Sanofi-Aventis; has received research grants by Biogen, Bayer-Schering, Merck, Teva and Novartis. Daniele Spitaleri received honoraria as a consultant on scientific advisory boards by Bayer-Schering, Novartis and Sanofi-Aventis and compensation for travel from Novartis, Biogen, Sanofi Aventis, Teva and Merck. Francesco Patti received personal compensation for serving on advisory board by Almirall, Alexion, Biogen, Bristol, Janssen, Merck, Novartis and Roche. He further received research grant by Alexion, Almirall, Biogen, Bristol, Merck, Novartis and Roche and by Fédération Internationale des Sociétés Magiques (FISM), Reload Association (Onlus), Italian Health Minister, and University of Catania. Davide Maimone received speaker honoraria for Advisory Board and travel grants from Alexion, Almirall, Bayer, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Matteo Foschi received travel and meeting attendance support from Novartis, Biogen, Roche, Sanofi-Genzyme and Merck. Andrea Surcinelli received travel and meeting attendance support from Novartis, Biogen, Roche, Merck, Bristol, Sanofi-Genzyme, Almirall, Piam. Bassem Yamout received honoraria as a speaker and member of scientific advisory boards from Sanofi, Bayer, Biogen, Merck, Janssen, Novartis, Roche and Aspen. Samia J. Khoury received compensation for scientific advisory board activity from Merck and Roche, and received compensation for serving on the IDMC for Biogen. Maria Jose Sa received consulting fees, speaker honoraria, and/or travel expenses for scientific meetings from Alexion, Bayer Healthcare, Biogen, Bristol Myers Squibb, Celgene, Janssen, Merck-Serono, Novartis, Roche, Sanofi and Teva. Cavit Boz received conference travel support from Biogen, Novartis, Bayer-Schering, Merck and Teva; has participated in clinical trials by Sanofi Aventis, Roche and Novartis. Bianca Weinstock-Guttman has participated in speaker's bureaus and/or served as a consultant for Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, Bayer, Janssen and Horizon. She also has received grant/research support from the agencies listed in the previous sentence. She serves in the editorial board for BMJ Neurology, Children, CNS Drugs, MS International and Frontiers in Epidemiology. Anneke van der Walt served on advisory boards for Novartis, Biogen, Merck and Roche and NervGen. She received unrestricted research grants from Novartis, Biogen, Merck and Roche. She is currently a co-Principal investigator on a co-sponsored observational study with Roche, evaluating a Roche-developed smartphone app, Floodlight-MS. She has received speaker's honoraria and travel support from Novartis, Roche, Biogen and Merck. She serves as the Chief Operating Officer of the MSBase Foundation (not-for-profit). Her primary research support is from the NHMRC of Australia and MS Research Australia. Vilija Jokubaitis receives research fellowship support from the National Health and Medical Research Council of Australia (2025360). Her institution receives research funding support from F. Hoffmann-La Roche, the International Progressive MS Alliance, Multiple Sclerosis Australia and the Pennycook Foundation outside of this current work. She has received speaker's honoraria from Novartis and The Limbic. Helmut Butzkueven's institution has received compensation for advisory boards or lecture fees from Novartis, Biogen, Merck, UCB Pharma and Roche. His institutions receive research funding from Novartis, Biogen, Merck, Roche, the NHMRC of Australia, The Medical Research Future Fund (Australia), Monash Partners, the Trish MS Foundation, The Pennycook Foundation, and MS Australia. He receives personal compensation as the Managing Director of the MSBase Foundation and from the Oxford Health Policy Forum Brain Health Initiative.
Figures


References
-
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: A review. JAMA. 2021;325(8):765–779. - PubMed
-
- Portaccio E, Bellinvia A, Fonderico M, et al. Progression is independent of relapse activity in early multiple sclerosis: A real-life cohort study. Brain. 2022;145(8):2796–2805. - PubMed
-
- Ransohoff RM. Multiple sclerosis: Role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol. 2023;44(4):266–275. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
