This is a preprint.
Nucleation feedback can drive establishment and maintenance of biased microtubule polarity in neurites
- PMID: 40909153
- PMCID: PMC12407633
Nucleation feedback can drive establishment and maintenance of biased microtubule polarity in neurites
Update in
-
Nucleation feedback can drive establishment and maintenance of biased microtubule polarity in neurites.Math Biosci. 2025 Nov;389:109538. doi: 10.1016/j.mbs.2025.109538. Epub 2025 Sep 22. Math Biosci. 2025. PMID: 40992539 Free PMC article.
Abstract
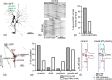
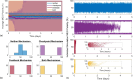
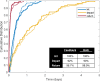
The microtubule cytoskeleton is comprised of dynamic, polarized filaments that facilitate transport within the cell. Polarized microtubule arrays are key to facilitating cargo transport in long cells such as neurons. Microtubules also undergo dynamic instability, where the plus and minus ends of the filaments switch between growth and shrinking phases, leading to frequent microtubule turnover. Although microtubules often completely disassemble and new filaments nucleate, microtubule arrays have been observed to both maintain their biased orientation throughout the cell lifetime and to rearrange their polarity as an adaptive response to injury. Motivated by cytoskeleton organization in neurites, we propose a spatially-explicit stochastic model of microtubule arrays and investigate how nucleation of new filaments could generate biased polarity in a simple linear domain. Using a continuous-time Markov chain model of microtubule growth dynamics, we model and parameterize two experimentally-validated nucleation mechanisms: nucleation feedback, where the direction of filament growth depends on existing microtubule content, and a checkpoint mechanism, where microtubules that nucleate in a direction opposite to the majority experience frequent catastrophe. When incorporating these validated mechanisms into the spatial model, we find that nucleation feedback is sufficient to establish biased polarity in neurites of different lengths, and that the emergence and maintenance of biased polarity is relatively stable in spite of stochastic fluctuations. This work provides a framework to study the relationship between microtubule nucleation and polarity, and could extend to give insights into mechanisms that drive the formation of polarized filament arrays in other biological settings.
Keywords: Stochastic simulation; microtubule turnover; nucleation feedback; parameterization.
Figures





References
-
- Bartolini F., Gundersen G. G., Generation of noncentrosomal microtubule arrays, Journal of cell science 119 (20) (2006) 4155–4163. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
