Functional ultrasound (fUS) detects mild cerebral alterations using canonical correlation analysis denoising and dynamic functional connectivity analysis
- PMID: 40909354
- PMCID: PMC12406050
- DOI: 10.1162/IMAG.a.128
Functional ultrasound (fUS) detects mild cerebral alterations using canonical correlation analysis denoising and dynamic functional connectivity analysis
Abstract
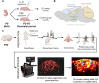
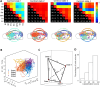
Functional ultrasound (fUS) is a promising imaging method for evaluating brain function in animals and human neonates. fUS images local cerebral blood volume changes to map brain activity. One application of fUS imaging is the quantification of functional connectivity (FC), which characterizes the strength of the connections between functionally connected brain areas. fUS-FC enables characterization of important cerebral alterations in pathological animal models, with potential for translation into identification of biomarkers of neurodevelopmental disorders. However, the sensitivity of fUS to signal sources other than cerebral activity, such as motion artifacts, cardiac pulsatility, anesthesia (if present), and respiration, limits its capacity to distinguish milder cerebral alterations. Here, we show that using canonical correlation analysis (CCA) preprocessing and dynamic functional connectivity analysis, we can efficiently decouple noise signals from the fUS-FC signal. We use this method to characterize the effects of a mild perinatal inflammation on FC in mice. The inflammation mouse model showed lower occurrence of states of high FC between the cortex, hippocampus, thalamus, and cerebellum as compared with controls, while connectivity states limited either to intracortical connections or to ventral pathways were more often observed in the inflammation model. These important differences could not be distinguished using other preprocessing techniques that we compared, such as global signal regression, highlighting the advantage of canonical correlation analysis for preprocessing fUS data. CCA preprocessing is applicable to a wide variety of fUS imaging experimental situations, from anesthetized to awake animal studies, or for neonatal, perinatal, or neurodevelopmental imaging. Beyond fUS imaging, this method can also be applied to FC data from any neuroimaging modality when the sources of noise can be spatially identified.
Keywords: canonical correlation analysis denoising; dynamic functional connectivity; functional ultrasound imaging; neuroinflammation; perinatal systemic inflammation.
© 2025 The Authors. Published under a Creative Commons Attribution 4.0 International (CC BY 4.0) license.
Conflict of interest statement
The authors have no competing interests to declare.
Figures




References
-
- Agut, T., Alarcon, A., Cabañas, F., Bartocci, M., Martinez-Biarge, M., Horsch, S., Agut, T., Alarcon, A., Arena, R., Bartocci, M., Bravo, M., Cabañas, F., Carreras, N., Claris, O., Dudink, J., Fumagalli, M., Govaert, P., Horsch, S., Parodi, A., … Valverde, E. (2020). Preterm white matter injury: Ultrasound diagnosis and classification. Pediatric Research, 87, 37–49. 10.1038/s41390-020-0781-1 - DOI - PMC - PubMed
-
- Aydin, A. K., Haselden, W. D., Goulam Houssen, Y., Pouzat, C., Rungta, R. L., Demené, C., Tanter, M., Drew, P. J., Charpak, S., & Boido, D. (2020). Transfer functions linking neural calcium to single voxel functional ultrasound signal. Nature Communications, 11(1), 1–10. 10.1038/s41467-020-16774-9 - DOI - PMC - PubMed
-
- Baranger, J., Demene, C., Frerot, A., Faure, F., Delanoë, C., Serroune, H., Houdouin, A., Mairesse, J., Biran, V., Baud, O., & Tanter, M. (2021). Bedside functional monitoring of the dynamic brain connectivity in human neonates. Nature Communications, 12(1), 1080. 10.1038/s41467-021-21387-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
