Impact of bleeding and thrombosis on outcome of 945 COVID-19 VV-ECMO cases from a German registry
- PMID: 40909455
- PMCID: PMC12405335
- DOI: 10.3389/fmed.2025.1649217
Impact of bleeding and thrombosis on outcome of 945 COVID-19 VV-ECMO cases from a German registry
Abstract
Bleeding and thromboembolic events (BTE) increase the mortality of COVID-19 acute respiratory distress syndrome (ARDS) treated with extracorporeal membrane oxygenation (ECMO). The current analysis aimed to assess frequency and determinants of BTE according to their location and severity in a retrospective analysis of the German ECMO COVID-19 registry. Logistic regression was applied to identify factors influencing ICU survival as well as variables associated with risks of BTE. In total, 708 of 945 patients (75%) suffered from BTE. Overall, 1,348 events were registered, including 406 (30%) major bleeding and 258 (19%) major thromboembolic events. Most common major bleeding locations were intracranial (n = 133, 10%) and pulmonary bleeding (n = 116, 9%). In-ICU survival was 35, 46% without BTE and 22% with major bleeding (p < 0.05). In summary, major bleeding was a core outcome-determinant of COVID-19 ECMO mortality with intracranial major bleeding as the most devastating complication (OR: 5.3; CI: 2.9-9.9; p < 0.001). Neither major thromboembolism nor minor BTE impacted ICU-mortality. Potentially modifiable factors associated with major bleeding included prolonged duration of ECMO >14 days (OR: 2.9; CI 1.8-4.7; p < 0.001) and platelet counts <100.000/μL ≥ 72 h (OR: 2.0; CI 1.1-3.6; p = 0.018). Hence, prevention, early recognition and treatment of major bleedings are key to increase the survival of COVID-19 ECMO. In this regard, our data indicate that the implementation of early weaning strategies to minimize duration of ECMO therapy and prevention of prolonged thrombocytopenia with platelet counts <100.000/μl ≥ 72 h could decrease the risk of devastating bleeds and could ameliorate survival.
Clinical trial registration: Registered in the German Clinical Trials Register (study ID: DRKS00022964), retrospectively registered, September 7th 2020, https://drks.de/DRKS00022964.
Keywords: COVID-19; acute respiraratory distress syndrome; bleeding; extracorporeal membrance oxygenation; thromboembolism.
Copyright © 2025 Herrmann, Schade, Meybohm, Paschke, Hübsch, Notz, Groene, Röder, Kranke, Merten, Landoll, Spieth, Kluge, Jarczak, Roedl, Sonntagbauer, Putensen, Schewe, Ehrentraut, Kreyer, Wehrfritz, Castellanos, Bihlmaier, Schmidt, Brenner, Herbstreit, Espeter, Wiefhoff, Ellerkmann, Oswald, Ellger, Lotz, Raimann, Wengenmayer, Staudacher, Zotzmann, Moerer, Kühn, Kochanek, Muellenbach, Glaser, Fichtner, Bodenstein, Findeisen, Rembold, Heim, Schneider, Lahmer, Padberg, Hullermann, Lepper, Becker, Danziger, Metz, Rosenberger, Mirakaj, Bernard, Braune, Roth, Grau, Heuschmann, Karagiannidis and Lotz.
Conflict of interest statement
PM reports honoraria for scientific lectures (Biotest AG, CSL Behring, Haemonetics, Pharmacosmos GmbH, Vifor Pharma). PK reports grants or contracts (Netzwerk Universitätsmedizin, APEPTICO GmbH, Federal Ministry of Education and Research (BMBF), Eagle Pharmaceutical); consulting fees (TEVA Ratiopharm, Amicus Ltd., Senzyme); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (TEVA Ratiopharm, CSL Vifor, Sintetica, CSL Behring, Senzyme, Fresenius Kabi, Pajunk); payment for expert testimony (Kanzlei Ulsenheimer & Friedrich Law Firm (München/Berlin), Landgericht Leipzig); support for attending meetings and/or travel [ASER (Prof. Dr. TJ Gan) for Consensus Meeting PONV 2024 Houston (Texas)]; participation on a data safety monitoring board or advisory board [Pharmacosmos, TEVA Ratiopharm, University Hospital Aachen (Tele-Emergency Medicine, Prof. Rolf Rossaint)]; leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid (Chairperson/Past chairperson of guideline committee ESAIC, Speaker of the Scientific Committee on Obstetric Anaesthesia within the German Society (DGAI), Member of Obstetric Committee within WFSA). DS reports consulting fees (Medtronic); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Getinge, AstraZeneca); support for attending meetings and/or travel (Abiomed, Getinge, Dahlhausen, Orinpharma, AstraZeneca, Medtronic). TB reports grants or contracts [Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Stiftung Universitätsmedizin Essen, Heidelberger Stiftung Chirurgie, Innovationsfonds des Gemeinsamen Bundesausschusses (G-BA)]; payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (CSL Behring GmbH, Schöchl medical education GmbH, Biotest AG, Baxter Deutschland GmbH, Boehringer Ingelheim Pharma GmbH, Astellas Pharma GmbH, B. Braun Melsungen AG; MSD Sharp & Dohme GmbH, Akademie für Infektionsmedizin e. v.; Lücke Kongresse GmbH, Pfizer Deutschland GmbH, MVZ Labor Dr. Limbach & Kollegen GbR); Patents planned issued or pending (BRAHMS GmbH); participation on a data safety monitoring board or advisory board (Baxter Deutschland GmbH, Bayer AG); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid (DGAI, DIVI, SepNet, DSG). MK reports stock or stock options (Biontech). FH reports payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (senior editor, Anesthesia and Analgesia); payment for expert testimony (in several court cases related to intensive care therapy); support for attending meetings and/or travel (various meetings, societies); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid [senior editor, Anesthesia & Analgesia; Auditor, German Society for Anesthesia and Intensive Care (DGAI)]. VM reports grants or contracts (DFG RO 1506/4-2). Peter Rosenberger reports grants or contracts (DFG RO 3671/14-1). CK reports grants or contracts (PI of X-COPD trial sponsored by Fresenius), consulting fees (Bayer, Xenios); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Bayer, Xenios, Getinge). Stefan Kluge reports grants or contracts (Cytosorbents, Daiichi Sankyo); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (ADVITOS, Biotest, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Shionogi and Zoll); participation on a data safety monitoring board or advisory board (ADVITOS, Fresenius, Gilead, MSD and Pfizer). KS reports grants or contracts [Stiftung Universitätsmedizin Essen (Microcirculation/Sepsis/Covid-19), Stiftung Chirurgie Heidelberg (Microcirculation/Shock), Ernst und Berta Grimmke Stiftung (Hyperspectral imaging/shock/machine learning)]; payment for expert testimony (expert witness in court). J-SP reports payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Abiomed Europe); support for attending meetings and/or travel (Abiomed Europe, Resuscitec). VZ reports grants or contracts (ELSO Grant for the study “Longterm outcome after COVID-ECMO-Therapy”). FF reports grants or contracts (CEOsys project funded by the Network of University Medicine [Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)] by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF), grant number 01KX2021). PH reports grants or contracts (German Ministry of Research and Education, European Union, German Parkinson Society, University Hospital Würzburg, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovation fond, German Research Foundation, Bavarian State, German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo); participation on a data safety monitoring board or advisory board [Participation on DSMB in publicly funded studies (by German Research Foundation, German Ministry of Research, Foundations)]. RM reports support for attending meetings and/or travel (Getinge). FR reports support for the present manuscript (publication fees from publication fund of the Goethe University); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (CSL Behring, King of Prussia, PA, USA, University hospital Wuerzburg, Germany, HELIOS clinics, Krefeld, Germany); support for attending meetings and/or travel (MCN congress organization, Nuernberg, Germany); patents planned issued or pending (Patent funding support from LifeSystems, Moenchengladbach, Germany). OM reports grants or contracts [research grant from Advitos for conducting experimental studies related to extracorporeal multi-organ support, member of the national CEOsys network Germany (Covid Ecosystem), and the Napkon-Tip (Therapeutic intervention platform for conducting ongoing assessments of new therapies), funded by the Federal Ministry of Education and Research (BMBF)]; royalties or licenses (honoraria from Springer, Book on neuro-monitoring in the ICU: Neuromonitoring in der Intensivmedizin 2023, ISBN: 978-3-662-65997-7); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Getinge Medical, CSL Behring, OM’s Department holds courses and workshops supported by companies related to Intensive Care Medicine); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid [German Society of Anesthesiology (DGAI)]. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
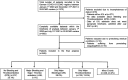
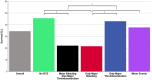
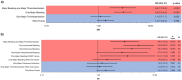
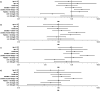
References
-
- Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in greater Paris, France: a multicentre cohort study. Lancet Respir Med. (2021) 9:851–62. doi: 10.1016/S2213-2600(21)00096-5, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

