This is a preprint.
Connectomics Reveals a Feed-Forward Swallowing Circuit Driving Protein Appetite
- PMID: 40909552
- PMCID: PMC12407811
- DOI: 10.1101/2025.08.25.671815
Connectomics Reveals a Feed-Forward Swallowing Circuit Driving Protein Appetite
Abstract
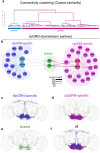
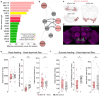
Nutrient state shapes not only what animals eat, but how they eat it. In Drosophila, protein deprivation prolongs protein-specific feeding bursts, yet the motor mechanism underlying this change remains unknown. Using EM connectomics, we identified a feed-forward pathway from protein-sensitive gustatory receptor neurons to swallowing motor neurons. At its core is the Sustain neuron, which coordinates multiple swallowing motor neurons to move food efficiently through the cibarium and pharynx. This nutrient-dependent facilitation of swallowing sustains long feeding bursts, directly linking internal state to the temporal structure of feeding. Our findings reveal how a dedicated sensorimotor circuit translates physiological need into precise motor control to drive nutrient specific feeding appetite.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
