This is a preprint.
An estrogen-independent and IL-1-dependent pathway controls vulvovaginal candidiasis through combined IL-17/IL-22 signaling
- PMID: 40909604
- PMCID: PMC12407711
- DOI: 10.1101/2025.08.25.671995
An estrogen-independent and IL-1-dependent pathway controls vulvovaginal candidiasis through combined IL-17/IL-22 signaling
Abstract
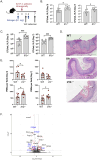
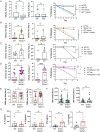
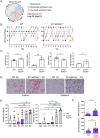
Vulvovaginal candidiasis (VVC), caused by the commensal pathobiont Candida albicans, affects >75% of women, marring quality of life and incurring significant health costs. Estrogen (E2) activity is tightly linked to VVC susceptibility, and preclinical models employ E2 to establish vaginal colonization. Unlike most forms of candidiasis, VVC is not considered to be a condition of immune compromise. Rather, VVC is characterized by high levels of PMNs and inflammatory cytokines that drive immunopathology but fail to clear the fungus. The role of the Type 17 pathway in this condition is controversial. Th17 signature profiles are upregulated in vaginal tissue during VVC in mice and humans. However, loss of individual Th17 components by gene deletion or anti-cytokine administration does not predispose to disease. Here, we reveal an IL-1/Type 17-driven arm of immunity that operates to control C. albicans in the vaginal mucosa independently of estrogen. Il1r -/- mice subjected to VVC bore high vaginal loads, accompanied by reduced IL-17A/F and IL-22 expression and suppressed PMN influx. Although mice lacking IL-17, IL-22/IL-22R or IL-23 individually exhibited normal susceptibility to VVC, mice lacking receptors for both cytokines (Il17raIl22ra1 -/-) had high and persistent fungal loads, with increased vaginal tissue damage and elevated IL-1α/β levels. Thus, IL-1R serves as a master regulator of protective Type 17 responses, and moreover IL-1 signaling alone is insufficient to control fungal colonization. Interestingly, Il1r -/- and Il17raIl22ra1 -/- mice showed high fungal colonization in the absence of exogenous estrogen, and this susceptibility persisted even when mice were given progesterone to prevent estrus. Together, these data reveal an estrogen-independent pathway of vaginal antifungal host defense mediated by combinatorial actions of IL-17 and IL-22 and governed by upstream IL-1R signaling.
Keywords: Candida albicans; IL-1; Th17; cytokine signaling; fungal immunity; mucosal candidiasis; vaginitis.
Figures


References
-
- Casalini G., Giacomelli A. & Antinori S. The WHO fungal priority pathogens list: a crucial reappraisal to review the prioritisation. Lancet Microbe 5, 717–724 (2024). - PubMed
-
- Lionakis M.S. & Levitz S.M. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annual review of immunology 36, 157–191 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
