This is a preprint.
Non-polio enteroviruses compromise the electrophysiology of a human iPSC-derived neural network
- PMID: 40909677
- PMCID: PMC12407919
- DOI: 10.1101/2025.08.27.672623
Non-polio enteroviruses compromise the electrophysiology of a human iPSC-derived neural network
Abstract
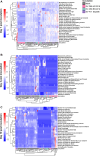
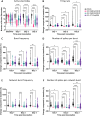
The non-polio enteroviruses enterovirus-D68 (EV-D68) and enterovirus-A71 (EV-A71) are highly prevalent and considered pathogens of increasing health concern. While most enterovirus infections are mild and self-limiting, severe complications ranging from meningitis, encephalitis, to acute flaccid paralysis can occur, especially in children and immunocompromised patients. Despite the global burden of neurological complications caused by EV-D68 and EV-A71, the underlying neuropathogenesis remains poorly understood. In particular, the impact of the infection on neural function has not been clearly elucidated. Here, we compare the replication kinetics, cellular tropism, and electrophysiological effects of EV-D68 and EV-A71 infection in a physiologically relevant human pluripotent stem cell-derived neural co-culture model, consisting of excitatory neurons and astrocytes. Inoculation with contemporary circulating EV-D68 strains and an EV-A71 strain resulted in decreased neural activity in the co-cultures, with EV-D68 A2/2018 inducing the most rapid and robust negative effect on neural co-cultures, followed by EV-D68 B3/2019. EV-D68 strains preferentially infected neurons, whereas EV-A71 infection was detected in both cell types to the same extent. Despite the lack of viral release of infectious virus particles of EV-D68 B3/2019 in the supernatant, the infection could spread in the cultures and reduce neurotransmission. Higher viral load and broader tropism of EV-A71 did not result in enhanced impairment of neural function. Our results demonstrate that neurotropic non-polio enteroviruses lead to disruption of spontaneous neural activity in a virus-specific manner, which does not correlate with their replication efficiency.
Keywords: micro-electrode array; neural networks; neurotropism; neurovirulence; non-polio enteroviruses.
Conflict of interest statement
Competing interests The authors report no competing interests.
Figures



References
-
- Harvala H, Johannesen CK, Benschop KSM, et al. Enterovirus circulation in the WHO European region, 2015–2022: a comparison of data from WHO’s three core poliovirus surveillance systems and the European Non-Polio Enterovirus Network (ENPEN). Lancet Reg Health Eur. 2025;53:101292. doi: 10.1016/j.lanepe.2025.101292 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
