This is a preprint.
FSP1 and histone deacetylases suppress cancer persister cell ferroptosis
- PMID: 40909720
- PMCID: PMC12407746
- DOI: 10.1101/2025.08.21.671520
FSP1 and histone deacetylases suppress cancer persister cell ferroptosis
Update in
-
FSP1 and histone deacetylases suppress cancer persister cell ferroptosis.Sci Adv. 2026 Jan 2;12(1):eaea8771. doi: 10.1126/sciadv.aea8771. Epub 2026 Jan 2. Sci Adv. 2026. PMID: 41481741 Free PMC article.
Abstract
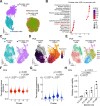
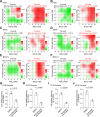
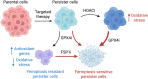
Cancer persister cells populate minimal residual disease and contribute to acquired drug resistance. We previously discovered that persister cells are sensitized to ferroptosis. However, our understanding of this emergent persister cell vulnerability remains limited, impeding ferroptosis drug development efforts. Here, we sought to understand key factors which govern persister cell ferroptosis to inform combinatorial treatment strategies. We found that persister cells can downregulate oxidative phosphorylation, a key source of reactive oxygen species, to avoid death from GPX4 inhibition. However, this can be overcome by pretreatment with clinically available histone deacetylase inhibitors which induce reactive oxygen species in persister cells and synergize with GPX4 inhibition. Furthermore, we found that while levels of iron, glutathione, and antioxidant genes are not universally dysregulated in persister cells, persister cells consistently downregulate alternative ferroptosis suppressor FSP1 and rely upon residual FSP1 to survive GPX4 inhibition. These findings reveal new strategies to eliminate persister cells by combining GPX4 inhibitors with histone deacetylase or FSP1 inhibitors.
Conflict of interest statement
Competing interests: MJH is a co-founder of Ferro Therapeutics, a subsidiary of BridgeBio Pharma, Inc.
Figures


References
-
- Russo M., Chen M., Mariella E., Peng H., Rehman S. K., Sancho E., Sogari A., Toh T. S., Balaban N. Q., Batlle E., Bernards R., Garnett M. J., Hangauer M., Leucci E., Marine J.-C., O’Brien C. A., Oren Y., Patton E. E., Robert C., Rosenberg S. M., Shen S., Bardelli A., Cancer drug-tolerant persister cells: from biological questions to clinical opportunities. Nat. Rev. Cancer 24, 694–717 (2024). - PMC - PubMed
-
- Terai H., Kitajima S., Potter D. S., Matsui Y., Quiceno L. G., Chen T., Kim T., Rusan M., Thai T. C., Piccioni F., Donovan K. A., Kwiatkowski N., Hinohara K., Wei G., Gray N. S., Fischer E. S., Wong K.-K., Shimamura T., Letai A., Hammerman P. S., Barbie D. A., ER Stress Signaling Promotes the Survival of Cancer “Persister Cells” Tolerant to EGFR Tyrosine Kinase Inhibitors. Cancer Res. 78, 1044–1057 (2018). - PMC - PubMed
-
- Viswanathan V. S., Ryan M. J., Dhruv H. D., Gill S., Eichhoff O. M., Seashore-Ludlow B., Kaffenberger S. D., Eaton J. K., Shimada K., Aguirre A. J., Viswanathan S. R., Chattopadhyay S., Tamayo P., Yang W. S., Rees M. G., Chen S., Boskovic Z. V., Javaid S., Huang C., Wu X., Tseng Y.-Y., Roider E. M., Gao D., Cleary J. M., Wolpin B. M., Mesirov J. P., Haber D. A., Engelman J. A., Boehm J. S., Kotz J. D., Hon C. S., Chen Y., Hahn W. C., Levesque M. P., Doench J. G., Berens M. E., Shamji A. F., Clemons P. A., Stockwell B. R., Schreiber S. L., Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
