Development of the insecticide oxazosulfyl
- PMID: 40910015
- PMCID: PMC12405013
- DOI: 10.1584/jpestics.J25-02
Development of the insecticide oxazosulfyl
Abstract
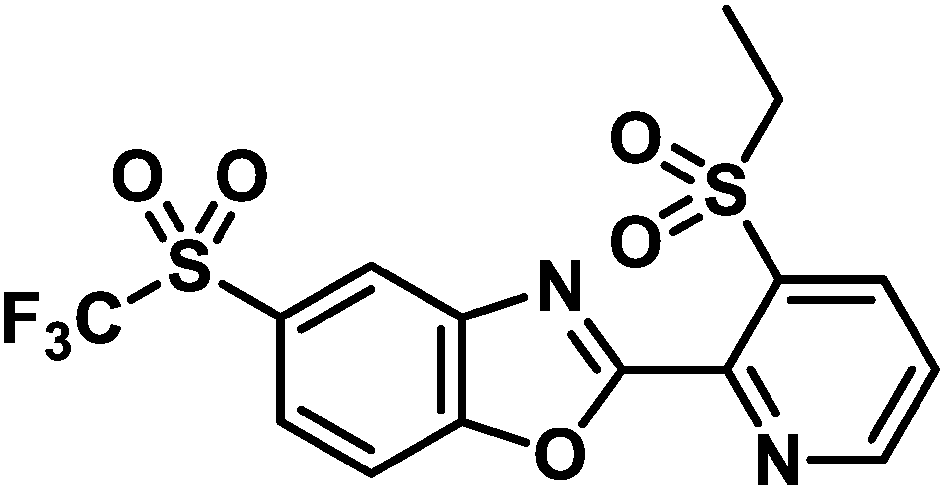
Oxazosulfyl, a novel insecticide originally discovered and developed by Sumitomo Chemical Co., Ltd., belongs to a new chemical class, the sulfyl group, structurally characterized by its ethylsulfonyl moiety. It exhibits excellent control against a broad range of major rice insect pests, including Coleoptera, Hemiptera, Lepidoptera, and Orthoptera, through nursery-box application. With a novel structural backbone and mode of action, this insecticide is classified by the Insecticide Resistance Action Committee as the sole member of novel code 37, vesicular acetylcholine transporter inhibitor. A substantial number of field studies in rice paddy fields have demonstrated that oxazosulfyl, registered in Japan in April 2021 as ALLES® granules, is highly effective against populations that have developed reduced sensitivity or resistance to existing insecticides. Given these favorable properties, oxazosulfyl is expected to contribute to the management of insecticide resistance, the reduction of agricultural chemical use, labor savings, and sustainable agriculture as a next-generation insecticide.
Keywords: broad spectrum; first-in-class; nursery box application; oxazosulfyl; resistance-breaker; sulfyl.
© 2025 Pesticide Science Society of Japan.
Figures






References
-
- 1) M. Matsumura and S. Sanada-Morimura: Recent status of insecticide resistance in Asian rice planthoppers. Jpn. Agric. Res. Q. 44, 225–230 (2010).
-
- 2) S. Sanada-Morimura, S. Sakumoto, R. Ohtsu, A. Otuka, S.-H. Huang, D. Van Thanh and M. Matsumura: Current status of insecticide resistance in the small brown planthopper, Laodelphax striatellus, in Japan, Taiwan, and Vietnam. Appl. Entomol. Zool. 46, 65–73 (2011).
-
- 3) M. Aoki: Control Effect of Some Insecticides and Resistance to Imidacloprid in Rice Leaf Beetle, Oulema oryzae in Hokkaido. Annual Report of the Society of Plant Protection of North Japan 66, 110–115 (2015) (in Japanese).
-
- 4) Y. Takahashi, H. Kikuchi and T. Niiyama: Control Effect of Some Insecticides and Resistance to Fipronil in Rice Leaf Beetle Oulema oryzae in Akita Prefecture. Annual Report of the Society of Plant Protection of North Japan 60, 174–176 (2009) (in Japanese).
-
- 5) M. Takahashi, T. Tanabe, M. Ito, A. Iwata and Y. Nokura (Sumitomo Chemical Co., Ltd.): Fused heterocyclic compounds and their use for pest control. Jpn. Pat. JP 5990057 B2 (2016).
LinkOut - more resources
Full Text Sources
Miscellaneous

