Characterization and Application of Novel Exercise Recovery Patterns That Reflect Cardiac Performance: A Substudy of the SEQUOIA-HCM Trial
- PMID: 40910168
- PMCID: PMC12493091
- DOI: 10.1161/CIRCULATIONAHA.124.073585
Characterization and Application of Novel Exercise Recovery Patterns That Reflect Cardiac Performance: A Substudy of the SEQUOIA-HCM Trial
Abstract
Background: Post-exercise oxygen uptake recovery (VO2Rec) is slow in advanced heart failure. We sought to establish easily derived VO2Rec measures and evaluate their cardiospecificity and prognostic relevance in patients with dyspnea on exertion. We further sought to determine VO2Rec modifiability proportional to changes in cardiac function with disease-specific treatment of obstructive hypertrophic cardiomyopathy.
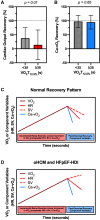
Methods: VO2Rec patterns were evaluated in relation to cardiac performance and the primary outcome of heart failure hospitalization or death in a referral cohort with dyspnea on exertion undergoing cardiopulmonary exercise testing with hemodynamic monitoring (MGH-ExS [Massachusetts General Hospital Exercise Study]). We then investigated longitudinal measures of VO2Rec in the pivotal phase 3 randomized controlled trial SEQUOIA-HCM (Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of Aficamten in Hypertrophic Cardiomyopathy) of aficamten versus placebo for 24 weeks in participants with symptomatic obstructive hypertrophic cardiomyopathy. For both cohorts, VO2Rec was uniformly measured as time for VO2 to decline by >0%, 12.5% (VO2T12.5%), 25%, and 50% of peak VO2.
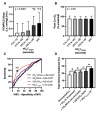
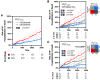
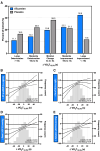
Results: Among 814 MGH-ExS patients (58±16 years of age, 58% women), those with a longer VO2T12.5% (≥35 versus <35 seconds) demonstrated elevated exercise pulmonary capillary wedge pressure to cardiac output slope (P<0.0001) with no difference in peripheral oxygen extraction (P=0.11). For each 15-second increase in VO2T12.5%, the hazard ratio for heart failure hospitalization and all-cause death was 1.54 (95% CI, 1.35-1.76; P<0.001). In SEQUOIA-HCM participants with cardiopulmonary exercise testing at baseline and week 24 (n=263, 59.1±2.9 years of age, 41% women), baseline VO2T12.5% was 45±20 seconds and improved 8 seconds (95% CI, -12 to -5 seconds; P<0.001) with aficamten treatment compared with placebo at 24 weeks. Participants treated with aficamten versus placebo were more likely to improve VO2T12.5% by ≥15 seconds (odds ratio [OR], 3.7 [95% CI, 1.9-6.9]; number needed to treat=4.8). Shortening of VO2T12.5% correlated with reduced NT-proBNP (N-terminal pro-B-type natriuretic peptide), high-sensitivity cardiac troponin I, and left ventricular outflow tract gradient (all P<0.005).
Conclusions: This study established VO2T12.5% as a new measure that reflects cardiac performance during exercise and predicted heart failure event-free survival. Furthermore, VO2T12.5% improved proportional to improvements in left ventricular outflow tract gradient and cardiac biomarkers in response to aficamten treatment, a cardiospecific therapy for obstructive hypertrophic cardiomyopathy. The simplicity and physiological relevance of VO2T12.5% support its regular inclusion in cardiopulmonary exercise testing protocols evaluating cardiac function during exercise.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT05186818.
Keywords: aficamten; cardiac myosin; cardiomyopathy, hypertrophic; exercise test; heart failure, diastolic; post-exercise recovery.
Conflict of interest statement
Dr Coats received speaker fees from Alnylam and Roche and advisory fees from Cytokinetics. Dr Lee received research grants through his institution from AstraZeneca, Boehringer Ingelheim, and Roche Diagnostics and is a member of a clinical end points committee for Bayer and a trial steering committee member for Cytokinetics. Dr Maron received consultant/advisor fees from Imbria, Edgewise, and BioMarin and steering committee fees for SEQUOIA-HCM from Cytokinetics. Dr Owens has received consulting/advisor fees from Bristol Myers Squibb and research grants from Bristol Myers Squibb, Cytokinetics, Novartis, and Actelion Pharmaceuticals. Dr Jacoby, Dr Heitner, Dr Kupfer, Dr Malik, and Dr Wohltman are employees and shareholders of Cytokinetics. Dr Malhotra is supported by the National Heart, Lung, and Blood Institute (R01HL142809, R01HL159514, and R01HL162928); the American Heart Association (22TPA969625); and the Wild Family Foundation. He receives research funding from Angea Biotherapeutics and Amgen and serves as a consultant for Pharmacosmos, Myokardia/BMS, Renovacor, Epizon Pharma, and Third Pole. He is a scientific cofounder of Angea Biotherapeutics and coinventor for a patent on pharmacologic BMP inhibitors (along with Mass General Brigham), for which he receives royalties. He receives royalties from UpToDate for scientific content authorship and received speaker bureau fees from Vox Media. Dr Lewis received research funding from the National Institutes of Health (R01-HL151841, R01-HL131029, and R01-HL159514), the American Heart Association Strategically Focused Research Network, Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, and SoniVie; honoraria for advisory boards from Pfizer, Merck, Boehringer Ingelheim, Novartis, American Regent, Cyclerion, Cytokinetics, and Amgen; and royalties from UpToDate for scientific content authorship related to exercise physiology.
Figures

References
-
- Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN, III, Davis SF, Wilson JR. Hemodynamic exercise testing: a valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94:3176–3183. doi: 10.1161/01.cir.94.12.3176 - PubMed
-
- Bailey CS, Wooster LT, Buswell M, Patel S, Pappagianopoulos PP, Bakken K, White C, Tanguay M, Blodgett JB, Baggish AL, et al. Post-exercise oxygen uptake recovery delay: a novel index of impaired cardiac reserve capacity in heart failure. JACC Heart Fail. 2018;6:329–339. doi: 10.1016/j.jchf.2018.01.007 - PMC - PubMed
-
- Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure: analysis with gas exchange measurements and NMR spectroscopy. Circulation. 1995;91:2924–2932. doi: 10.1161/01.cir.91.12.2924 - PubMed
-
- Coats CJ, Maron MS, Abraham TP, Olivotto I, Lee MMY, Arad M, Cardim N, Ma CS, Choudhury L, Dungen HD, et al. ; SEQUOIA-HCM Investigators. Exercise capacity in patients with obstructive hypertrophic cardiomyopathy: SEQUOIA-HCM baseline characteristics and study design. JACC Heart Fail. 2024;12:199–215. doi: 10.1016/j.jchf.2023.10.004 - PubMed
-
- Maron MS, Masri A, Nassif ME, Barriales-Villa R, Arad M, Cardim N, Choudhury L, Claggett B, Coats CJ, Dungen HD, et al. ; SEQUOIA-HCM Investigators. Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N Engl J Med. 2024;390:1849–1861. doi: 10.1056/NEJMoa2401424 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

