The Organization of Central Retinal Projections in Anna's Hummingbirds (Calypte anna) and Zebra Finches (Taeniopygia castanotis)
- PMID: 40911558
- PMCID: PMC12413023
- DOI: 10.1002/cne.70087
The Organization of Central Retinal Projections in Anna's Hummingbirds (Calypte anna) and Zebra Finches (Taeniopygia castanotis)
Abstract
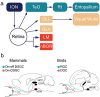
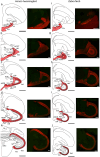
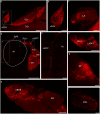
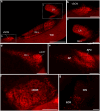
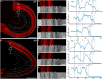
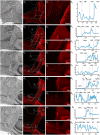
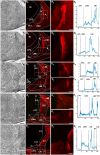
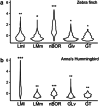
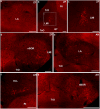
Hummingbirds (family Trochilidae) are easily recognized due to their unique ability to hover. Critical to hovering flight is head and body stabilization. In birds, stabilization during flight is mediated, among other things, by the detection of optic flow, the motion that occurs across the entire retina during self-motion. Given this increased requirement for stabilization, it is not surprising that previous studies have shown that hummingbirds have neural specializations in the visual pathways involved in the detection of optic flow. Particularly, previous studies have found some structural and functional differences in the hummingbird brain, in the pretectal nucleus lentiformis mesencephali (LM): compared to other avian species, LM shows a massive hypertrophy, and LM neurons have unique response properties to optic flow stimuli. Here, we used intraocular injections of a neural tracer, cholera toxin subunit B (CTB) conjugated with a fluorescent molecule, to study the retinal projections in Anna's hummingbirds (Calypte anna) and compare them to those of a similarly sized non-hovering species, the zebra finch (Taeniopygia castanotis). Retinal targets in both birds were similar and correspond closely to those reported in other birds from a variety of avian clades. Importantly, we found differences in the projections to LM between hummingbirds and zebra finches. Consistent with previous reports of specialization of LM, it was more intensely labelled compared to other retinal-recipient nuclei in hummingbirds. Moreover, this increase in intensity was most apparent in the lateral subnucleus. This study reinforces previous evidence that the LM of hummingbirds is adapted to sustain the unique flight abilities of this clade.
Keywords: avian; optic flow; optic tectum; retina; visual system.
© 2025 The Author(s). The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Altshuler, D. L. , and Dudley R.. 2002. “The Ecological and Evolutionary Interface of Hummingbird Flight Physiology.” Journal of Experimental Biology 205: 2325–2336. - PubMed
-
- Beer, G. D 1974. Homology, an Unsolved Problem. Carolina Biological Supply Company.
-
- Bellintani‐Guardia, B. , and Ott M.. 2002. “Displaced Retinal Ganglion Cells Project to the Accessory Optic System in the Chameleon (Chamaeleo calyptratus).” Experimental Brain Research 145: 56–63. - PubMed
-
- Benowitz, L. I. , and Karten H. J.. 1976. “Organization of the Tectofugal Visual Pathway in the Pigeon: A Retrograde Transport Study.” Journal of Comparative Neurology 167: 503–520. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

